WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Morphology and Structure
2.2. TG-DTA (Thermogravimetric-Differential Thermal Analysis) and XPS Analysis
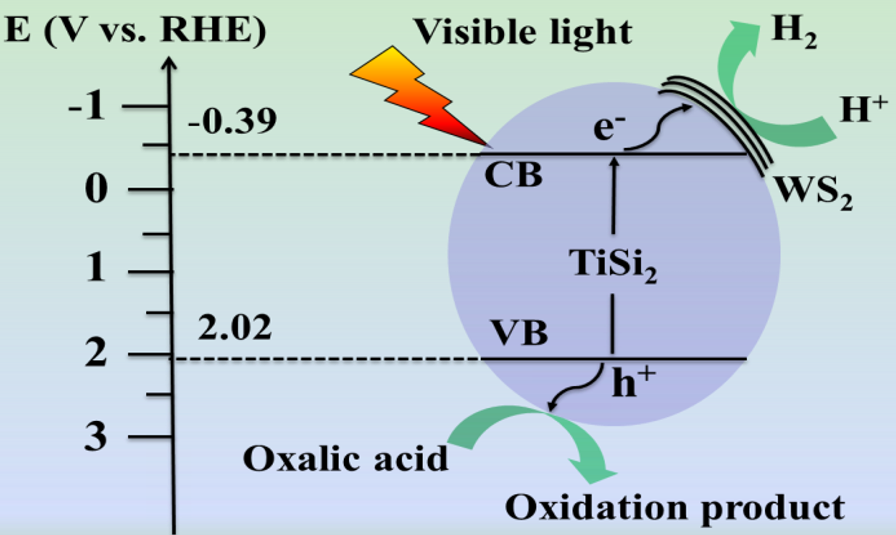
2.3. Optical and Photoelectrochemical Properties
2.4. Photocatalytic Hydrogen Evolution
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
3.3. Photoelectrochemical Measurements
3.4. Photocatalytic Reaction
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sato, J.; Kobayashi, H.; Ikarashi, K.; Saito, N.; Nishiyama, H.; Inoue, Y. Photocatalytic Activity for Water Decomposition of RuO2-Dispersed Zn2GeO4 with d10 Configuration. J. Phys. Chem. B 2004, 108, 4369–4375. [Google Scholar] [CrossRef]
- Ingler, W.B.; Baltrus, J.P.; Khan, S.U.M. Photoresponse of p-Type Zinc-Doped Iron(III) Oxide Thin Films. J. Am. Chem. Soc. 2004, 126, 10238–10239. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-H.; Huang, C.-W.; Wu, J.C.S. Hydrogen Production from Semiconductor-based Photocatalysis via Water Splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Lin, Y.J.; Xu, Y.; Mayer, M.T.; Simpson, Z.I.; McMahon, G.; Zhou, S.; Wang, D.W. Growth of p-Type Hematite by Atomic Layer Deposition and Its Utilization for Improved Solar Water Splitting. J. Am. Chem. Soc. 2012, 134, 5508–5511. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.; Parida, K.M.; Singh, S.K. Facile Fabrication of S-TiO2/β-SiC Nanocomposite Photocatalyst for Hydrogen Evolution under Visible Light Irradiation. ACS Sustain. Chem. Eng. 2015, 3, 245–253. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Woan, K.; Pyrgiotakis, G.; Sigmund, W. Photocatalytic Carbon-Nanotube—TiO2 Composites. Adv. Mater. 2009, 21, 2233–2239. [Google Scholar] [CrossRef]
- Yang, P.; Lu, C.; Hua, N.P.; Du, Y.K. Titanium dioxide nanoparticles co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater. Lett. 2002, 57, 794–801. [Google Scholar] [CrossRef]
- Maeda, K.; Takata, T.; Hara, M.; Saito, N.; Inoue, Y.; Kobayashi, H.; Domen, K. GaN:ZnO Solid Solution as a Photocatalyst for Visible-Light-Driven Overall Water Splitting. J. Am. Chem. Soc. 2005, 127, 8286–8287. [Google Scholar] [CrossRef] [PubMed]
- Pany, S.; Parida, K.M. Sulfate-Anchored Hierarchical Meso-Macroporous N-doped TiO2: A Novel Photocatalyst for Visible Light H2 Evolution. ACS Sustain. Chem. Eng. 2014, 2, 1429–1438. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Liu, N.Y.; Han, Y.Z.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.-T.; Zhong, J.; Kang, Z.H. Metal-free efficient photocatalyst for stable visible water splitting via a two-electron pathway. Science 2015, 347, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, J.L.; Rowe, J.E.; Nemanich, R.J. Titanium silicide islands on atomically clean Si(100): Identifying single electron tunneling effects. J. Appl. Phys. 2010, 107, 123715. [Google Scholar] [CrossRef]
- Ritterskamp, P.; Kuklya, A.; Wüstkamp, M.-A.; Kerpen, K.; Weidenthaler, C.; Demuth, M. A Titanium Disilicide Derived Semiconducting Catalyst for Water Splitting under Solar Radiation—Reversible Storage of Oxygen and Hydrogen. Angew. Chem. Int. Ed. 2007, 46, 7770–7774. [Google Scholar] [CrossRef] [PubMed]
- Mou, Z.G.; Yin, S.L.; Zhu, M.S.; Wang, X.M.; Zheng, J.W.; Lu, C.; Du, Y.K.; Yang, P. RuO2/TiSi2/graphene composite for enhanced photocatalytic hydrogen generation under visible light irradiation. Phys. Chem. Chem. Phys. 2013, 15, 2793–2799. [Google Scholar] [CrossRef] [PubMed]
- Lingampalli, S.R.; Gautam, U.K.; Rao, C.N.R. Highly efficient photocatalytic hydrogen generation by solution-processed ZnO/Pt/CdS, ZnO/Pt/Cd1−xZnxS and ZnO/Pt/CdS1-xSex hybrid nanostructures. Energy Environ. Sci. 2013, 6, 3589–3594. [Google Scholar] [CrossRef]
- Wang, Y.B.; Wang, Y.S.; Xu, R. Photochemical Deposition of Pt on CdS for H2 Evolution from Water: Markedly Enhanced Activity by Controlling Pt Reduction Environment. J. Phys. Chem. C 2013, 117, 783–790. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Lu, Z-H.; Chen, X.S. Ultrafine Ni-Pt Alloy Nanoparticles Grown on Graphene as Highly Efficient Catalyst for Complete Hydrogen Generation from Hydrazine Borane. ACS Sustain. Chem. Eng. 2015, 3, 1255–1261. [Google Scholar] [CrossRef]
- Zong, X.; Yan, H.J.; Wu, G.P.; Ma, G.J.; Wen, F.Y.; Wang, L.; Li, C. Enhancement of Photocatalytic H2 Evolution on CdS by Loading MoS2 as Cocatalyst under Visible Light Irradiation. J. Am. Chem. Soc. 2008, 130, 7176–7177. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Xie, S.F.; Li, H.; Wang, X.Y. A Highly Efficient Sunlight Driven ZnO Nanosheet Photocatalyst: Synergetic Effect of P-Doping and MoS2 Atomic Layer Loading. ChemCatChem 2014, 6, 2522–2526. [Google Scholar] [CrossRef]
- Chen, G.P.; Li, D.M.; Li, F.; Fan, Y.Z.; Zhao, H.F.; Luo, Y.H.; Yu, R.C.; Meng, Q.B. Ball-milling combined calcination synthesis of MoS2/CdS photocatalysts for high photocatalytic H2 evolution activity under visible light irradiation. Appl. Catal. A Gen. 2012, 443, 138–144. [Google Scholar] [CrossRef]
- Xiang, Q.J.; Yu, J.G.; Jaroniec, M. Synergetic Effect of MoS2 and Graphene as Cocatalysts for Enhanced Photocatalytic H2 Production Activity of TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.T.; Wang, D.D.; Yang, P.; Du, Y.K.; Lu, C. Coupling ZnxCd1−xS nanoparticles with graphenelike MoS2: Superior interfacial contact, low overpotential and enhanced photocatalytic activity under visible-light irradiation. Catal. Sci. Technol. 2014, 4, 2650–2657. [Google Scholar] [CrossRef]
- Jing, D.W.; Guo, L.J. WS2 sensitized mesoporous TiO2 for efficient photocatalytic hydrogen production from water under visible light irradiation. Catal. Commun. 2007, 8, 795–799. [Google Scholar] [CrossRef]
- Zong, X.; Han, J.F.; Ma, G.J.; Yan, H.J.; Wu, G.P.; Li, C. Photocatalytic H2 Evolution on CdS Loaded with WS2 as Cocatalyst under Visible Light Irradiation. J. Phys. Chem. C 2011, 115, 12202–12208. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Fang, B.Z.; Bonakdarpour, A.; Sun, A.K.; Wilkinson, D.P.; Wang, D.Z. WS2 nanosheets as a highly efficient electrocatalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2012, 125, 59–66. [Google Scholar] [CrossRef]
- Chen, G.P.; Li, F.; Fan, Y.Z.; Luo, Y.H.; Li, D.M.; Meng, Q.B. A novel noble metal-free ZnS-WS2/CdS composite photocatalyst for H2 evolution under visible light irradiation. Catal. Commun. 2013, 40, 51–54. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, W.J.; Gong, Q.F.; Liu, C.H.; Liu, Z.; Li, Y.G.; Dai, H.J. Ultrathin WS2 Nanoflakes as a High-Performance Electrocatalyst for the Hydrogen Evolution Reaction. Angew. Chem. Int. Ed. 2014, 53, 7860–7863. [Google Scholar] [CrossRef] [PubMed]
- Adriano, A.; Zdenêk, S.; Martin, P. 2H→1T phase transition and hydrogen evolution activity of MoS2, MoSe2, WS2 and WSe2 strongly depends on the MX2 composition. Chem. Commun. 2015, 51, 8450–8453. [Google Scholar]
- Yen, B.K. X-ray diffraction study of solid-state formation of metastable MoSi2 and TiSi2 during mechanical alloying. J. Appl. Phys. 1997, 81, 7061–7063. [Google Scholar] [CrossRef]
- An, G.J.; Chai, Y.M.; Zhong, H.J.; Zhang, C.F.; Liu, C.G. Thermal decompositon behavior of ammonium tertrathiotungstate under nitrogen atmosphere. Abstr. Pap. Am. Chem. Soc. 2005, 230, U2321–U2322. [Google Scholar]
- Bhandavat, R.; David, L.; Singh, G. Synthesis of Surface-Functionalized WS2 Nanosheets and Performance as Li-ion Battery Anodes. J. Phys. Chem. Lett. 2012, 3, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.-P.; Ma, J.; Luo, J.-M.; Yu, J.; He, J.K.; Meng, Y.T.; Luo, Z.; Bao, S.-K.; Liu, H.-L.; Luo, S.-L.; et al. Fabrication of novel heterostructured few layered WS2-Bi2WO6/Bi3.84W0.16O6.24 composites with enhanced photocatalytic performance. Appl. Catal. B 2015, 179, 220–228. [Google Scholar] [CrossRef]
- Frame, F.A.; Osterloh, F.E. CdSe-MoS2: A Quantum Size-Confined Photocatalyst for Hydrogen Evolution from Water under Visible Light. J. Phys. Chem. C 2010, 114, 10628–10633. [Google Scholar] [CrossRef]
- Meng, F.K.; Li, J.T.; Cushing, S.K.; Zhi, M.J.; Wu, N.Q. Solar Hydrogen Generation by Nanoscale p-n Junction of p-type Molybdenum Disulfide/n-type Nitrogen-Doped Reduced Graphene Oxide. J. Am. Chem. Soc. 2013, 135, 10286–10289. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, Q.; Xiao, X.D. Hydrogen Evolution from Pt Nanoparticles Covered p-Type CdS:Cu Photocathode in Scavenger-Free Electrolyte. J. Phys. Chem. C 2014, 118, 2306–2311. [Google Scholar] [CrossRef]
- Cardo, F.; Gomes, W.P. On the determination of the flat-band potential of a semiconductor in contact with a metal or an electrolyte from the Mott-Schottky plot. J. Phys. D Appl. Phys. 1978, 11, L63–L67. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Fu, H.-B.; Zhu, Y.-F. Efficient TiO2 Photocatalysts from Surface Hybridization of TiO2 Particles with Graphite-like Carbon. Adv. Funct. Mater. 2008, 18, 2180–2189. [Google Scholar] [CrossRef]
- Kaneka, H.; Nishimoto, S.; Miyake, K.; Suedomi, N. Physical and electrochemichromic properties of rf sputtered tungsten oxide films. J. Appl. Phys. 1986, 59, 2526–2534. [Google Scholar] [CrossRef]
- Al-Gaashani, R.; Radiman, S.; Tabet, N.; Daud, A.R. Rapid synthesis and optical properties of hematite (α-Fe2O3) nanostructures using a simple thermal decomposition method. J. Alloys Compd. 2013, 550, 395–401. [Google Scholar] [CrossRef]
- Park, Y.; Kang, S.-H.; Choi, W.Y. Exfoliated and reorganized graphite oxide on titania nanoparticles as an auxiliary co-catalyst for photocatalytic solar conversion. Phys. Chem. Chem. Phys. 2011, 13, 9425–9431. [Google Scholar] [CrossRef] [PubMed]
- He, B.-L.; Dong, B.; Li, H.-L. Preparation and electrochemical properties of Ag-modified TiO2 nanotube anode material for lithium-ion battery. Electrochem. Commun. 2007, 9, 425–430. [Google Scholar] [CrossRef]
- Li, Q.Y.; Lu, G.X. Significant Effect of Pressure on the H2 Releasing from Photothermal-Catalytic Water Steam Splitting over TiSi2 and Pt/TiO2. Catal. Lett. 2008, 125, 376–379. [Google Scholar] [CrossRef]
- Thomazeau, C.; Geantet, C.; Lacroix, M.; Harle, V.; Benazeth, S.; Marhic, C.; Danot, M. Two Cation Disulfide Layers in the WxMo(1−x)S2 Lamellar Solid Solution. J. Solid State Chem. 2001, 160, 147–155. [Google Scholar] [CrossRef]
- Liu, J.J.; Bai, Y.N.; Chen, P.W.; Cui, N.F.; Yin, H. Reaction synthesis of TiSi2 and Ti5Si3 by ball-milling and shock loading and their photocatalytic activities. J. Alloys Compd. 2013, 555, 375–380. [Google Scholar] [CrossRef]









© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, D.; Zhang, C.; Yang, P.; Du, Y.; Lu, C. WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2016, 6, 136. https://doi.org/10.3390/catal6090136
Chu D, Zhang C, Yang P, Du Y, Lu C. WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts. 2016; 6(9):136. https://doi.org/10.3390/catal6090136
Chicago/Turabian StyleChu, Dongmei, Chunyong Zhang, Ping Yang, Yukou Du, and Cheng Lu. 2016. "WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation" Catalysts 6, no. 9: 136. https://doi.org/10.3390/catal6090136
APA StyleChu, D., Zhang, C., Yang, P., Du, Y., & Lu, C. (2016). WS2 as an Effective Noble-Metal Free Cocatalyst Modified TiSi2 for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts, 6(9), 136. https://doi.org/10.3390/catal6090136