Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalyst Characterization
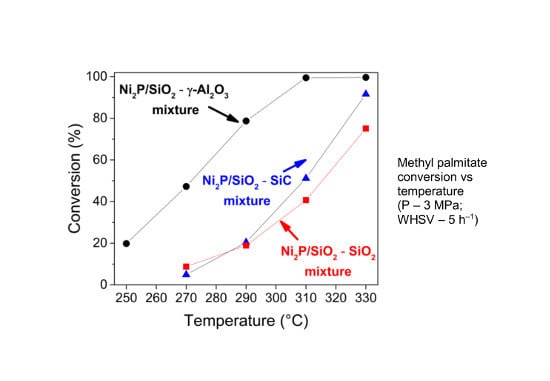
2.2. Hydrodeoxygenation of Methyl Palmitate over Ni2P/SiO2 Catalyst
3. Experimental
3.1. Materials
3.2. Catalyst Synthesis
3.3. Catalyst Characterization
3.4. Catalytic Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kay Lup, A.N.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A review on reactivity and stability of heterogeneous metal catalysts for deoxygenation of bio-oil model compounds. J. Ind. Eng. Chem. 2017. [Google Scholar] [CrossRef]
- Si, Z.; Zhang, X.; Wang, C.; Ma, L.; Dong, R. An overview on catalytic hydrodeoxygenation of pyrolysis oil and its model compounds. Catalysts 2017, 7, 169. [Google Scholar] [CrossRef]
- Choudhary, T.V.; Phillips, C.B. Renewable fuels via catalytic hydrodeoxygenation. Appl. Catal. A 2011, 397, 1–12. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Ramos-Fernandez, E.V.; Sepulveda-Escribano, A. From biodiesel and bioethanol to liquid hydrocarbon fuels: New hydrotreating and advanced microbial technologies. Energy Environ. Sci. 2012, 5, 5638–5652. [Google Scholar] [CrossRef]
- Hachemi, I.; Jenistova, K.; Maki-Arvela, P.; Kumar, N.; Eranen, K.; Hemming, J.; Murzin, D.Y. Comparative study of sulfur-free nickel and palladium catalysts in hydrodeoxygenation of different fatty acid feedstocks for production of biofuels. Catal. Sci. Technol. 2016, 6, 1476–1487. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C. Biomass conversion technologies: Catalytic conversion technologies. In Biorefineries: Targeting Energy, High Value Products and Waste Valorisation; Rabaçal, M., Ferreira, A.F., Silva, C.A.M., Costa, M., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 113–121. [Google Scholar]
- Kay Lup, A.N.; Abnisa, F.; Daud, W.M.A.W.; Aroua, M.K. A review on reaction mechanisms of metal-catalyzed deoxygenation process in bio-oil model compounds. Appl. Catal. A 2017, 541, 87–106. [Google Scholar] [CrossRef]
- Li, D.; Xin, H.; Du, X.; Hao, X.; Liu, Q.; Hu, C. Recent advances for the production of hydrocarbon biofuel via deoxygenation progress. Sci. Bull. 2015, 60, 2096–2106. [Google Scholar] [CrossRef]
- Mikulec, J.; Cvengros, J.; Jorikova, L.; Banic, M.; Kleinova, A. Second generation diesel fuel from renewable sources. J. Clean. Prod. 2010, 18, 917–926. [Google Scholar] [CrossRef]
- Hari, T.K.; Yaakob, Z. Production of diesel fuel by the hydrotreatment of jatropha oil derived fatty acid methyl esters over gamma-Al2O3 and SiO2 supported NiCo bimetallic catalysts. React. Kinet. Mech. Catal. 2015, 116, 131–145. [Google Scholar] [CrossRef]
- Hari, T.K.; Yaakob, Z. CoFe/gamma-Al2O3 Catalyst for the hydrotreatment of fatty acid methyl esters (FAME). Chem. Lett. 2015, 44, 1237–1239. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; Chen, J.W. Hydroprocessing of biomass-derived oils and their blends with petroleum feedstocks: A review. Energy Fuels 2012, 26, 5373–5399. [Google Scholar] [CrossRef]
- Kanda, Y.; Matsukura, Y.; Sawada, A.; Sugioka, M.; Uemichi, Y. Low-temperature synthesis of rhodium phosphide on alumina and investigation of its catalytic activity toward the hydrodesulfurization of thiophene. Appl. Catal. A 2016, 515, 25–31. [Google Scholar] [CrossRef]
- Guan, Q.X.; Wan, F.F.; Han, F.; Liu, Z.H.; Li, W. Hydrodeoxygenation of methyl palmitate over MCM-41 supported nickel phosphide catalysts. Catal. Today 2016, 259, 467–473. [Google Scholar] [CrossRef]
- Kubicka, D.; Kaluza, L. Deoxygenation of vegetable oils over sulfided Ni, Mo and NiMo catalysts. Appl. Catal. A 2010, 372, 199–208. [Google Scholar] [CrossRef]
- Donnis, B.; Egeberg, R.G.; Blom, P.; Knudsen, K.G. Hydroprocessing of bio-oils and oxygenates to hydrocarbons. Understanding the reaction routes. Top. Catal. 2009, 52, 229–240. [Google Scholar] [CrossRef]
- Simacek, P.; Kubicka, D.; Kubickova, I.; Homola, F.; Pospisil, M.; Chudoba, J. Premium quality renewable diesel fuel by hydroprocessing of sunflower oil. Fuel 2011, 90, 2473–2479. [Google Scholar] [CrossRef]
- Lapuerta, M.; Villajos, M.; Agudelo, J.R.; Boehman, A.L. Key properties and blending strategies of hydrotreated vegetable oil as biofuel for diesel engines. Fuel Process. Technol. 2011, 92, 2406–2411. [Google Scholar] [CrossRef]
- Furimsky, E. Hydroprocessing challenges in biofuels production. Catal. Today 2013, 217, 13–56. [Google Scholar] [CrossRef]
- Laurent, E.; Delmon, B. Study of the hydrodeoxygenation of carbonyl, carboxylic and guaiacyl groups over sulfided como/gamma-Al2O3 and nimo/gamma-Al2O3 catalyst. 2. Influence of water, ammonia and hydrogen-sulfide. Appl. Catal. A 1994, 109, 97–115. [Google Scholar] [CrossRef]
- Senol, O.I.; Viljava, T.R.; Krause, A.O.I. Hydrodeoxygenation of methyl esters on sulphided NiMo/gamma-Al2O3 and CoMo/gamma-Al2O3 catalysts. Catal. Today 2005, 100, 331–335. [Google Scholar] [CrossRef]
- Senol, O.I.; Ryymin, E.-M.; Viljava, T.-R.; Krause, A.O.I. Reactions of methyl heptanoate hydrodeoxygenation on sulphided catalysts. J. Mol. Catal. Chem. 2007, 268, 1–8. [Google Scholar] [CrossRef]
- Coumans, A.E.; Hensen, E.J.M. A real support effect on the hydrodeoxygenation of methyl oleate by sulfided NiMo catalysts. Catal. Today 2017. [Google Scholar] [CrossRef]
- Itthibenchapong, V.; Srifa, A.; Kaewmeesri, R.; Kidkhunthod, P.; Faungnawakij, K. Deoxygenation of palm kernel oil to jet fuel-like hydrocarbons using Ni-MoS2/γ-Al2O3 catalysts. Energy Convers. Manag. 2017, 134, 188–196. [Google Scholar] [CrossRef]
- Bie, Y.W.; Gutierrez, A.; Viljava, T.R.; Kanervo, J.M.; Lehtonen, J. Hydrodeoxygenation of methyl heptanoate over noble metal catalysts: Catalyst screening and reaction network. Ind. Eng. Chem. Res. 2013, 52, 11544–11551. [Google Scholar] [CrossRef]
- Ma, B.; Zhao, C. High-grade diesel production by hydrodeoxygenation of palm oil over a hierarchically structured Ni/HBEA catalyst. Green Chem. 2015, 17, 1692–1701. [Google Scholar] [CrossRef]
- Kon, K.; Onodera, W.; Takakusagi, S.; Shimizu, K. Hydrodeoxygenation of fatty acids and triglycerides by Pt-loaded Nb2O5 catalysts. Catal. Sci. Technol. 2014, 4, 3705–3712. [Google Scholar] [CrossRef]
- Madsen, A.T.; Ahmed, E.; Christensen, C.H.; Fehrmann, R.; Riisager, A. Hydrodeoxygenation of waste fat for diesel production: Study on model feed with Pt/alumina catalyst. Fuel 2011, 90, 3433–3438. [Google Scholar] [CrossRef]
- Goto, H.; Takagaki, A.; Kikuchi, R.; Oyama, S.T. Hydrogenation of 2,5-dimethylfuran on hexagonal-boron nitride- and silica-supported platinum catalysts. Appl. Catal. A 2017. [Google Scholar] [CrossRef]
- Senol, O.I.; Ryymin, E.M.; Viljava, T.R.; Krause, A.O.I. Effect of hydrogen sulphide on the hydrodeoxygenation of aromatic and aliphatic oxygenates on sulphided catalysts. J. Mol. Catal. Chem. 2007, 277, 107–112. [Google Scholar] [CrossRef]
- Pinheiro, A.; Dupassieux, N.; Hudebine, D.; Geantet, C. Impact of the presence of carbon monoxide and carbon dioxide on gas oil hydrotreatment: Investigation on liquids from biomass cotreatment with petroleum cuts. Energy Fuels 2011, 25, 804–812. [Google Scholar] [CrossRef]
- Philippe, M.; Richard, F.; Hudebine, D.; Brunet, S. Transformation of dibenzothiophenes model molecules over CoMoP/Al2O3 catalyst in the presence of oxygenated compounds. Appl. Catal. B 2013, 132, 493–498. [Google Scholar] [CrossRef]
- Vlasova, E.N.; Deliy, I.V.; Nuzhdin, A.L.; Aleksandrov, P.V.; Gerasimov, E.Y.; Aleshina, G.I.; Bukhtiyarova, G.A. Catalytic properties of CoMo/Al2O3 sulfide catalysts in the hydrorefining of straight-run diesel fraction mixed with rapeseed oil. Kinet. Catal. 2014, 55, 481–491. [Google Scholar] [CrossRef]
- Ochoa-Hernandez, C.; Yang, Y.X.; Pizarro, P.; O’Shea, V.A.D.; Coronado, J.M.; Serrano, D.P. Hydrocarbons production through hydrotreating of methyl esters over Ni and Co supported on SBA-15 and Al-SBA-15. Catal. Today 2013, 210, 81–88. [Google Scholar] [CrossRef]
- Loe, R.; Santillan-Jimenez, E.; Morgan, T.; Sewell, L.; Ji, Y.Y.; Jones, S.; Isaacs, M.A.; Lee, A.F.; Crocker, M. Effect of Cu and Sn promotion on the catalytic deoxygenation of model and algal lipids to fuel-like hydrocarbons over supported Ni catalysts. Appl. Catal. B 2016, 191, 147–156. [Google Scholar] [CrossRef]
- Kukushkin, R.G.; Bulavchenko, O.A.; Kaichev, V.V.; Yakovlev, V.A. Influence of Mo on catalytic activity of Ni-based catalysts in hydrodeoxygenation of esters. Appl. Catal. B 2015, 163, 531–538. [Google Scholar] [CrossRef]
- Yakovlev, V.A.; Khromova, S.A.; Sherstyuk, O.V.; Dundich, V.O.; Ermakov, D.Y.; Novopashina, V.M.; Lebedev, M.Y.; Bulavchenko, O.; Parmon, V.N. Development of new catalytic systems for upgraded bio-fuels production from bio-crude-oil and biodiesel. Catal. Today 2009, 144, 362–366. [Google Scholar] [CrossRef]
- Zuo, H.L.; Liu, Q.Y.; Wang, T.J.; Ma, L.L.; Zhang, O.; Zhang, Q. Hydrodeoxygenation of methyl palmitate over supported Ni catalysts for diesel-like fuel production. Energy Fuels 2012, 26, 3747–3755. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, R.; Chen, J. Deoxygenation of methyl laurate as a model compound on Ni-Zn alloy and intermetallic compound catalysts: Geometric and electronic effects of oxophilic Zn. Appl. Catal. B 2017. [Google Scholar] [CrossRef]
- Wang, H.Y.; Jiao, T.T.; Li, Z.X.; Li, C.S.; Zhang, S.J.; Zhang, J.L. Study on palm oil hydrogenation for clean fuel over Ni-Mo-W/gamma-Al2O3-ZSM-5 catalyst. Fuel Process. Technol. 2015, 139, 91–99. [Google Scholar] [CrossRef]
- Hollak, S.A.W.; Gosselink, R.W.; van Es, D.S.; Bitter, J.H. Comparison of tungsten and molybdenum carbide catalysts for the hydrodeoxygenation of oleic acid. ACS Catal. 2013, 3, 2837–2844. [Google Scholar] [CrossRef]
- Wang, H.L.; Yan, S.L.; Salley, S.O.; Ng, K.Y.S. Support effects on hydrotreating of soybean oil over NiMo carbide catalyst. Fuel 2013, 111, 81–87. [Google Scholar] [CrossRef]
- Monnier, J.; Sulimma, H.; Dalai, A.; Caravaggio, G. Hydrodeoxygenation of oleic acid and canola oil over alumina-supported metal nitrides. Appl. Catal. A 2010, 382, 176–180. [Google Scholar] [CrossRef]
- Chen, J.X.; Shi, H.; Li, L.; Li, K.L. Deoxygenation of methyl laurate as a model compound to hydrocarbons on transition metal phosphide catalysts. Appl. Catal. B 2014, 144, 870–884. [Google Scholar] [CrossRef]
- Chen, J.X.; Yang, Y.; Shi, H.; Li, M.F.; Chu, Y.; Pan, Z.Y.; Yu, X.B. Regulating product distribution in deoxygenation of methyl laurate on silica-supported Ni-Mo phosphides: Effect of Ni/Mo ratio. Fuel 2014, 129, 1–10. [Google Scholar] [CrossRef]
- Shi, H.; Chen, J.X.; Yang, Y.; Tian, S.S. Catalytic deoxygenation of methyl laurate as a model compound to hydrocarbons on nickel phosphide catalysts: Remarkable support effect. Fuel Process. Technol. 2014, 118, 161–170. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, J.X.; Shi, H. Deoxygenation of methyl laurate as a model compound to hydrocarbons on Ni2P/SiO2, Ni2P/MCM-41, and Ni2P/SBA-15 catalysts with different dispersions. Energy Fuels 2013, 27, 3400–3409. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ochoa-Hernandez, C.; O’Shea, V.A.D.; Coronado, J.M.; Serrano, D.P. Ni2P/SBA-15 As a hydrodeoxygenation catalyst with enhanced selectivity for the conversion of methyl oleate into n-octadecane. ACS Catal. 2012, 2, 592–598. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ochoa-Hernandez, C.; Pizarro, P.; O’Shea, V.A.D.; Coronado, J.M.; Serrano, D.P. Synthesis of nickel phosphide nanorods as catalyst for the hydrotreating of methyl oleate. Top. Catal. 2012, 55, 991–998. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ochoa-Hernandez, C.; Pizarro, P.; O’Shea, V.A.D.; Coronado, J.M.; Serrano, D.P. Influence of the Ni/P ratio and metal loading on the performance of NixPy/SBA-15 catalysts for the hydrodeoxygenation of methyl oleate. Fuel 2015, 144, 60–70. [Google Scholar] [CrossRef]
- Zarchin, R.; Rabaev, M.; Vidruk-Nehemya, R.; Landau, M.V.; Herskowitz, M. Hydroprocessing of soybean oil on nickel-phosphide supported catalysts. Fuel 2015, 139, 684–691. [Google Scholar] [CrossRef]
- Pan, Z.Y.; Wang, R.J.; Li, M.F.; Chu, Y.; Chen, J.X. Deoxygenation of methyl laurate to hydrocarbons on silica-supported Ni-Mo phosphides: Effect of calcination temperatures of precursor. J. Energy Chem. 2015, 24, 77–86. [Google Scholar] [CrossRef]
- Xue, Y.A.; Guan, Q.X.; Li, W. Synthesis of bulk and supported nickel phosphide using microwave radiation for hydrodeoxygenation of methyl palmitate. RSC Adv. 2015, 5, 53623–53628. [Google Scholar] [CrossRef]
- Peroni, M.; Lee, I.; Huang, X.; Barath, E.; Gutiérrez, O.Y.; Lercher, J.A. Deoxygenation of palmitic acid on unsupported transition metal phosphides. ACS Catal. 2017. [Google Scholar] [CrossRef]
- Zheng, Z.; Li, M.F.; Chu, Y.; Chen, J.X. Influence of CS2 on performance of Ni2P/SiO2 for deoxygenation of methyl laurate as a model compound to hydrocarbons: Simultaneous investigation on catalyst deactivation. Fuel Process. Technol. 2015, 134, 259–269. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Pakharukova, V.P.; Gerasimov, E.Y.; Rogov, V.A.; Bukhtiyarova, G.A. Effect of the preparation conditions on the physicochemical and catalytic properties of Ni2P/SiO2 catalysts. Russ. Chem. Bull. 2015, 64, 2361–2370. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Deliy, I.V.; Aleksandrov, P.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Ayupov, A.B.; Andreev, A.S.; Lapina, O.B.; Bukhtiyarova, G.A. Effect of precursor on the catalytic properties of Ni2P/SiO2 in methyl palmitate hydrodeoxygenation. RSC Adv. 2016, 6, 30372–30383. [Google Scholar] [CrossRef]
- Alvarez-Galvan, M.C.; Blanco-Brieva, G.; Capel-Sanchez, M.; Morales-delaRosa, S.; Campos-Martin, J.M.; Fierro, J.L.G. Metal phosphide catalysts for the hydrotreatment of non-edible vegetable oils. Catal. Today 2017. [Google Scholar] [CrossRef]
- Liu, X.; Li, Z.; Zhang, B.; Hu, M. Improvement of hydrodeoxygenation stability of nickel phosphide based catalysts by silica modification as structural promoter. Fuel 2017, 204, 144–151. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Z.; Zhu, K.; Chen, J. Hydroconversion of methyl laurate on bifunctional Ni2P/AlMCM-41 catalyst prepared via in situ phosphorization using triphenylphosphine. Appl. Surf. Sci. 2017, 404, 388–397. [Google Scholar] [CrossRef]
- Xin, H.; Guo, K.; Li, D.; Yang, H.; Hu, C. Production of high-grade diesel from palmitic acid over activated carbon-supported nickel phosphide catalysts. Appl. Catal. B 2016, 187, 375–385. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, R.; Nie, Z.; Chen, J. Effect of a second metal (Co, Fe, Mo and W) on performance of Ni2P/SiO2 for hydrodeoxygenation of methyl laurate. J. Energy Chem. 2016, 25, 418–426. [Google Scholar] [CrossRef]
- Guan, Q.X.; Han, F.; Li, W. Catalytic performance and deoxygenation path of methyl palmitate on Ni2P/SiO2 synthesized using the thermal decomposition of nickel hypophosphite. RSC Adv. 2016, 6, 31308–31315. [Google Scholar] [CrossRef]
- Chen, J.; Han, M.; Zhao, S.; Pan, Z.; Zhang, Z. An in situ approach to preparing Ni2P/SiO2 catalyst under mild conditions and its performance for the deoxygenation of methyl laurate to hydrocarbons. Catal. Sci. Technol. 2016, 6, 3938–3949. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, M.; Chen, J. Effects of P/Ni ratio and Ni content on performance of γ-Al2O3-supported nickel phosphides for deoxygenation of methyl laurate to hydrocarbons. Appl. Surf. Sci. 2016, 360 Pt A, 353–364. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Yang, H.; Jing, Z.-Y.; Xi, K.-Z.; Qiao, C.-Z. Hydrodeoxygenation of fatty acid methyl esters and isomerization of products over NiP/SAPO-11 catalysts. J. Fuel Chem. Technol. 2016, 44, 1211–1216. [Google Scholar] [CrossRef]
- Luo, N.; Cao, Y.; Li, J.; Guo, W.; Zhao, Z.-W. Preparation of Ni2P/Zr-MCM-41 catalyst and its performance in the hydrodeoxygenation of Jatropha curcas oil. J. Fuel Chem. Technol. 2016, 44, 76–83. [Google Scholar] [CrossRef]
- Lee, S.II.; Kim, D.W.; Jeon, H.J.; Ju, S.J.; Ryu, J.W.; Oh, S.H.; Koh, J.H. Metal Phosphorus Compound for Preparing Biodiesel and Method Preparing Biodiesel Using the Same. Patent No. US2014/0150332A1, 5 June 2014. [Google Scholar]
- Gosselink, R.W.; Hollak, S.A.W.; Chang, S.W.; van Haveren, J.; de Jong, K.P.; Bitter, J.H.; van Es, D.S. Reaction pathways for the deoxygenation of vegetable oils and related model compounds. ChemSusChem 2013, 6, 1576–1594. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.W.; O’Connor, P.; Corma, A. Processing biomass in conventional oil refineries: Production of high quality diesel by hydrotreating vegetable oils in heavy vacuum oil mixtures. Appl. Catal. A 2007, 329, 120–129. [Google Scholar] [CrossRef]
- Deliy, I.V.; Vlasova, E.N.; Nuzhdin, A.L.; Gerasimov, E.Y.; Bukhtiyarova, G.A. Hydrodeoxygenation of methyl palmitate over sulfided Mo/Al2O3, CoMo/Al2O3 and NiMo/Al2O3 catalysts. RSC Adv. 2014, 4, 2242–2250. [Google Scholar] [CrossRef]
- Prins, R.; Bussell, M.E. Metal phosphides: preparation, characterization and catalytic reactivity. Catal. Lett. 2012, 142, 1413–1436. [Google Scholar] [CrossRef]
- Liu, X.G.; Xu, L.; Zhang, B.Q. Essential elucidation for preparation of supported nickel phosphide upon nickel phosphate precursor. J. Solid State Chem. 2014, 212, 13–22. [Google Scholar] [CrossRef]
- Peroni, M.; Mancino, G.; Baráth, E.; Gutiérrez, O.Y.; Lercher, J.A. Bulk and γ‑Al2O3-supported Ni2P and MoP for hydrodeoxygenation of palmitic acid. Appl. Catal. B 2016, 180, 301–311. [Google Scholar] [CrossRef]
- Oyama, S.; Wang, X.; Lee, Y.; Bando, K.; Requejo, F. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques. J. Catal. 2002, 210, 207–217. [Google Scholar] [CrossRef]
- Cecilia, J.; Infantes-Molina, A.; Rodriguez-Castellon, E.; Jimenez-Lopez, A. A novel method for preparing an active nickel phosphide catalyst for HDS of dibenzothiophene. J. Catal. 2009, 263, 4–15. [Google Scholar] [CrossRef]
- Stinner, C.; Prins, R.; Weber, T. Binary and ternary transition-metal phosphides as HDN catalysts. J. Catal. 2001, 202, 187–194. [Google Scholar] [CrossRef]
- Berhault, G.; Afanasiev, P.; Loboue, H.; Geantet, C.; Cseri, T.; Pichon, C.; Guillot-Deudon, C.; Lafond, A. In Situ XRD, XAS, and magnetic susceptibility study of the reduction of ammonium nickel phosphate NiNH4PO4 center dot H2O into nickel phosphide. Inorg. Chem. 2009, 48, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Stinner, C.; Tang, Z.; Haouas, M.; Weber, T.; Prins, R. Preparation and P-31 NMR characterization of nickel phosphides on silica. J. Catal. 2002, 208, 456–466. [Google Scholar] [CrossRef]
- Liu, D.P.; Wang, A.J.; Liu, C.G.; Prins, R. Bulk and Al2O3-supported Ni2P HDS catalysts prepared by separating the nickel and hypophosphite sources. Catal. Commun. 2016, 77, 13–17. [Google Scholar] [CrossRef]
- Li, D.P.; Wang, A.J.; Liu, C.G.; Prins, R. Ni2P/Al2O3 hydrodesulfurization catalysts prepared by separating the nickel compound and hypophosphite. Catal. Today 2017, 292, 133–142. [Google Scholar] [CrossRef]
- Wu, S.K.; Lai, P.C.; Lin, Y.C. Atmospheric Hydrodeoxygenation of guaiacol over nickel phosphide catalysts: Effect of phosphorus composition. Catal. Lett. 2014, 144, 878–889. [Google Scholar] [CrossRef]
- Lee, Y.K.; Oyama, S.T. Bifunctional nature of a SiO2-supported Ni2P catalyst for hydrotreating: EXAFS and FTIR studies. J. Catal. 2006, 239, 376–389. [Google Scholar] [CrossRef]
- Cecilia, J.A.; Infantes-Molina, A.; Rodriguez-Castellon, E.; Jimenez-Lopez, A.; Oyama, S.T. Oxygen-removal of dibenzofuran as a model compound in biomass derived bio-oil on nickel phosphide catalysts: Role of phosphorus. Appl. Catal. B 2013, 136, 140–149. [Google Scholar] [CrossRef]
- Berteau, P.; Delmon, B. Modified aluminas—Relationship between activity in 1-butanol dehydration and acidity measured by NH3 TPD. Catal. Today 1989, 5, 121–137. [Google Scholar] [CrossRef]
- Kijeński, J.; Baiker, A. Acidic sites on catalyst surfaces and their determination. Catal. Today 1989, 5, 1–120. [Google Scholar] [CrossRef]
- Turek, W.; Haber, J.; Krowiak, A. Dehydration of isopropyl alcohol used as an indicator of the type and strength of catalyst acid centres. Appl. Surf. Sci. 2005, 252, 823–827. [Google Scholar] [CrossRef]
- Han, F.; Guan, Q.; Li, W. Deoxygenation of methyl palmitate over SiO2-supported nickel phosphide catalysts: Effects of pressure and kinetic investigation. RSC Adv. 2015, 5, 107533–107539. [Google Scholar] [CrossRef]
- Liu, Y.H.; Yao, L.; Xin, H.; Wang, G.S.; Li, D.; Hu, C.W. The production of diesel-like hydrocarbons from palmitic acid over HZSM-22 supported nickel phosphide catalysts. Appl. Catal. B 2015, 174, 504–514. [Google Scholar] [CrossRef]
- Prins, R. Hydrogen spillover. facts and fiction. Chem. Rev. 2012, 112, 2714–2738. [Google Scholar] [CrossRef] [PubMed]
- Fenelonov, V.B.; Romannikov, V.N.; Derevyankin, A.Y. Mesopore size and surface area calculations for hexagonal mesophases (types MCM-41, FSM-16, etc.) using low-angle XRD and adsorption data. Microporous Mesoporous Mater. 1999, 28, 57–72. [Google Scholar] [CrossRef]
- International Centre for Diffration Data (JCPDS); Fachinformationszentrum (FIZ): Karlsruhe, Germany, 2011.










| Sample | Ni (wt %) | Ni/P Molar Ratio (from EDX 2) | DTEM (nm) |
|---|---|---|---|
| NiPxOy/SiO2 | 2.6 ± 0.3 | - | - |
| reduced ex situ Ni2P/SiO2 | 2.5 ± 0.2 | 1.5 ± 0.1 | 3.6 ± 0.8 |
| Spent 1 Ni2P/SiO2 | 2.5 ± 0.2 | 1.6 ± 0.1 | 3.3 ± 0.7 |
| Sample | Treduction, °C | NH3-TPD | |
|---|---|---|---|
| Tmax, °C | Quantity, μmol/g | ||
| SiO2 | - | 231 | 84 |
| Ni2P/SiO2 | 600 | 231 | 110 |
| γ-Al2O3 | - | 237 | 106 |
| 335 | 315 | ||
| Characteristics | SiC | SiO2 | γ-Al2O3 |
|---|---|---|---|
| Shape | Grains | sphere | cylinder |
| Size of granules (mm) | 0.1–0.2 | 4 | 4 × 1.5 |
| SBET (m2/g) | 1 | 300 | 235 |
| Dpore (nm) | - | 10.6 | 13.4 |
| Pore volume (cm3/g) | - | 0.80 | 0.79 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamanaev, I.V.; Deliy, I.V.; Gerasimov, E.Y.; Pakharukova, V.P.; Kodenev, E.G.; Aleksandrov, P.V.; Bukhtiyarova, G.A. Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate. Catalysts 2017, 7, 329. https://doi.org/10.3390/catal7110329
Shamanaev IV, Deliy IV, Gerasimov EY, Pakharukova VP, Kodenev EG, Aleksandrov PV, Bukhtiyarova GA. Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate. Catalysts. 2017; 7(11):329. https://doi.org/10.3390/catal7110329
Chicago/Turabian StyleShamanaev, Ivan V., Irina V. Deliy, Evgeny Yu. Gerasimov, Vera P. Pakharukova, Evgeny G. Kodenev, Pavel V. Aleksandrov, and Galina A. Bukhtiyarova. 2017. "Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate" Catalysts 7, no. 11: 329. https://doi.org/10.3390/catal7110329
APA StyleShamanaev, I. V., Deliy, I. V., Gerasimov, E. Y., Pakharukova, V. P., Kodenev, E. G., Aleksandrov, P. V., & Bukhtiyarova, G. A. (2017). Synergetic Effect of Ni2P/SiO2 and γ-Al2O3 Physical Mixture in Hydrodeoxygenation of Methyl Palmitate. Catalysts, 7(11), 329. https://doi.org/10.3390/catal7110329