Aqueous Phase Hydrogenolysis of Bio-Derivable Furfuryl Alcohol to Pentanediols Using Copper Catalysts
Abstract
:1. Introduction
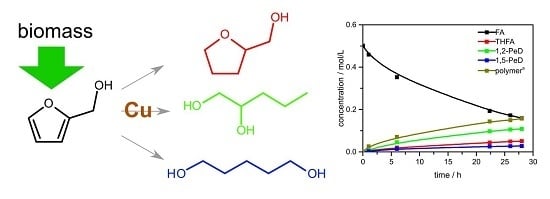
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Claus, P.; Vogel, H. The Roll of Chemocatalysis in the Establishment of the Technology Platform “Renewable Resources”. Chem. Eng. Technol. 2008, 31, 678–699. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Reduction of Biomass-Derived Furanic Compounds with Hydrogen. ACS Catal. 2013, 3, 2655–2668. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007. [Google Scholar]
- Nakagawa, Y.; Tamura, M.; Tomishige, K. Catalytic Conversions of Furfural to Pentanediols. Catal. Surv. Asia 2015, 19, 249–256. [Google Scholar] [CrossRef]
- Werle, P.; Morawietz, M.; Lundmark, S.; Sorensen, K.; Karvinen, E.; Lehtonen, J. Alcohols, Polyhydric. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar]
- Agostini, I.; Cupferman, S. Cosmetic Composition Comprising an Aqueous Dispersion of Film-Forming Polymer Particles Containing 1,2-pentanediol. US 6296858B1, 2 October 2001. [Google Scholar]
- Siegmeier, R.; Prescher, G.; Maurer, H.; Hering, G. Kontinuierliches Verfahren zur Herstellung von 1,2-Pentandiol. EP 0183029 A3, 5 August 1987. [Google Scholar]
- Fu, J.; Wang, J.; Sun, C.; Zhang, D.; Wu, Z. Method for Preparing 1,2-pentanediol. CN 103864575 A, 18 June 2014. [Google Scholar]
- Kaufmann, W.E.; Adams, R. The Use of Platinum Oxide as a Catalyst in the Reduction of Organic Compounds. IV. Reduction of Furfural and Its Derivatives. J. Am. Chem. Soc. 1923, 45, 3029–3044. [Google Scholar] [CrossRef]
- Adkins, H.; Connor, R. The Catalytic Hydrogenation of Organic Compounds over Copper Chromite. J. Am. Chem. Soc. 1931, 53, 1091–1095. [Google Scholar] [CrossRef]
- Connor, R.; Adkins, H. Hydrogenolysis of Oxygenated Organic Compounds. J. Am. Chem. Soc. 1932, 54, 4678–4690. [Google Scholar] [CrossRef]
- Connor, R.; Folkers, K.; Adkins, H. The preparation of copper-chromium oxide catalysts for hydrogenation. J. Am. Chem. Soc. 1932, 54, 1138–1145. [Google Scholar] [CrossRef]
- Zhang, B.; Zhu, Y.; Ding, G.; Zheng, H.; Li, Y. Selective conversion of furfuryl alcohol to 1,2-pentanediol over a Ru/MnOx catalyst in aqueous phase. Green Chem. 2012, 14, 3402–3409. [Google Scholar] [CrossRef]
- Khan, F.-A.; Vallat, A.; Suss-Fink, G. Highly selective low-temperature hydrogenation of furfuryl alcohol to tetrahydrofurfuryl alcohol catalysed by hectorite-supported ruthenium nanoparticles. Catal. Commun. 2011, 12, 1428–1431. [Google Scholar] [CrossRef]
- Marianne, O.; Neumann, M.; Brehme, V.; Christoph, T.; Dorit, W.; Claus, P.; Lucas, M.; Eckert, R. Hydrogenolyse von Furfurylalkohol zu 1,2-Pentandiol. DE102013203420 A1, 28 August 2014. [Google Scholar]
- Gotz, D.; Lucas, M.; Claus, P. C–O bond hydrogenolysis vs. C=C group hydrogenation of furfuryl alcohol: Towards sustainable synthesis of 1,2-pentanediol. React. Chem. Eng. 2016, 1, 161–164. [Google Scholar] [CrossRef]
- Nishimura, S. Hydrogenation and Hydrogenolysis. V. Rhodium-Platinum Oxide as a Catalyst for the Hydrogenation of Organic Compounds. Bull. Chem. Soc. Jpn. 1961, 34, 32–36. [Google Scholar] [CrossRef]
- Smith, H.A.; Fuzek, J.F. Catalytic Hydrogenation of Furan and Substituted Furans on Platinum. J. Am. Chem. Soc. 1949, 71, 415–419. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Liu, X.; Ren, J.; Wang, Y.; Lu, G. Direct catalytic conversion of furfural to 1,5-pentanediol by hydrogenolysis of the furan ring under mild conditions over Pt/CO2AlO4 catalyst. Chem. Commun. 2011, 47, 3924–3926. [Google Scholar] [CrossRef] [PubMed]
- Mizugaki, T.; Yamakawa, T.; Nagatsu, Y.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. Direct Transformation of Furfural to 1,2-Pentanediol Using a Hydrotalcite-Supported Platinum Nanoparticle Catalyst. ACS Sustain. Chem. Eng. 2014, 2, 2243–2247. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Kang, H.; Xia, C.; Chen, J. Selective hydrogenolysis of biomass-derived furfuryl alcohol into 1,2- and 1,5-pentanediol over highly dispersed Cu-Al2O3 catalysts. Chin. J. Catal. 2016, 37, 700–710. [Google Scholar] [CrossRef]
- Liu, H.; Huang, Z.; Zhao, F.; Cui, F.; Li, X.; Xia, C.; Chen, J. Efficient hydrogenolysis of biomass-derived furfuryl alcohol to 1,2- and 1,5-pentanediols over a non-precious Cu-Mg3AlO4.5 bifunctional catalyst. Catal. Sci. Technol. 2016, 6, 668–671. [Google Scholar] [CrossRef]
- Bienholz, A.; Hofmann, H.; Claus, P. Selective hydrogenolysis of glycerol over copper catalysts both in liquid and vapour phase: Correlation between the copper surface area and the catalyst‘s activity. Appl. Catal. A 2011, 391, 153–157. [Google Scholar] [CrossRef]


| Cat. # | Catalyst Composition (%) | X a (%) | 1,2-PeD (%) | 1,5-PeD (%) | 1,4-PeD (%) | THFA (%) | Others (%) | Sum. (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 60 CuO 40 ZnO | 12 | 16 | 5 | 2 | 22 | 13 | 58 |
| 2 | 47 CuO 46 Cr2O3 7 BaO | 8 | 5 | 1 | 1 | 25 | 12 | 44 |
| 3 | 40 CuO 40 ZnO 20 Al2O3 | 13 | 16 | 4 | 2 | 19 | 10 | 51 |
| 4 | 70 CuO 30 SiO2 | 14 | 2 | 0 | 1 | 8 | 5 | 16 |
| 5 | 89 CuO 9 SiO2 2 CaO | 6 | 6 | 2 | 1 | 24 | 9 | 42 |
| 6 | 36 CuO 48 ZnO 16 Al2O3 | 16 | 27 | 8 | 3 | 12 | 10 | 60 |
| 7 | 53 CuO 47 MgO/SiO2 | 13 | 9 | 4 | 2 | 29 | 9 | 53 |
| 8 | 50 CuO 20 ZrO2 30 ZnO | 13 | 23 | 8 | 2 | 25 | 11 | 69 |
| 9 | 60 CuO 40 ZrO2 | 22 | 26 | 8 | 2 | 18 | 8 | 62 |
| 9 b | 60 CuO 40 ZrO2 | 100 | 0 | 0 | 0 | 0 | 44 | 44 |
| - c | - | 5 | 5 | 1 | 1 | 25 | 7 | 39 |
| Cat. # a | Copper Specific Reaction Rate (10−2 mmol·min−1·gCu−1) | Copper Surface (m2·gcat.−1) from [24] | |
|---|---|---|---|
| 1,2-PeD | THFA | ||
| 1 | 0.64 | 0.82 | 16.2 |
| 2 | 0.10 | 0.63 | 12.0 |
| 3 | 1.01 | 0.94 | 4.7 |
| 4 | 0.03 | 0.22 | 8.5 |
| 5 | 0.06 | 0.28 | 5.1 |
| 6 | 3.09 | 1.06 | 11.9 |
| 7 | 0.51 | 1.47 | - |
| 8 | 1.26 | 1.19 | - |
| 9 | 2.21 | 1.39 | - |
| Heat-up Gas | X b (%) | 1,2-PeD (%) | THFA (%) | 1,5-PeD (%) | 1,4-PeD (%) | Others (%) | Sum. (%) |
|---|---|---|---|---|---|---|---|
| hydrogen | 22 | 26 | 18 | 8 | 2 | 8 | 62 |
| argon | 21 | 18 | 14 | 5 | 3 | 10 | 50 |
| argon a | 33 | 18 | 24 | 5 | 2 | 6 | 55 |
| hydrogen a | 32 | 28 | 12 | 9 | 2 | 6 | 57 |
| T/K | pH2 (MPa) | X a (%) | 1,2-PeD (%) | 1,5-PeD (%) | 1,4-PeD (%) | THFA (%) | Others (%) | Sum. (%) |
|---|---|---|---|---|---|---|---|---|
| 443 | 5 | 22 | 26 | 8 | 2 | 18 | 8 | 62 |
| 443 | 7 | 24 | 30 | 9 | 3 | 18 | 7 | 67 |
| 443 | 9 | 28 | 34 | 10 | 3 | 18 | 10 | 75 |
| 473 | 5 | 54 | 10 | 2 | 3 | 7 | 35 | 57 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Götz, D.; Lucas, M.; Claus, P. Aqueous Phase Hydrogenolysis of Bio-Derivable Furfuryl Alcohol to Pentanediols Using Copper Catalysts. Catalysts 2017, 7, 50. https://doi.org/10.3390/catal7020050
Götz D, Lucas M, Claus P. Aqueous Phase Hydrogenolysis of Bio-Derivable Furfuryl Alcohol to Pentanediols Using Copper Catalysts. Catalysts. 2017; 7(2):50. https://doi.org/10.3390/catal7020050
Chicago/Turabian StyleGötz, Dominik, Martin Lucas, and Peter Claus. 2017. "Aqueous Phase Hydrogenolysis of Bio-Derivable Furfuryl Alcohol to Pentanediols Using Copper Catalysts" Catalysts 7, no. 2: 50. https://doi.org/10.3390/catal7020050
APA StyleGötz, D., Lucas, M., & Claus, P. (2017). Aqueous Phase Hydrogenolysis of Bio-Derivable Furfuryl Alcohol to Pentanediols Using Copper Catalysts. Catalysts, 7(2), 50. https://doi.org/10.3390/catal7020050