Oxidation Catalysis by Enzymes in Microemulsions
Abstract
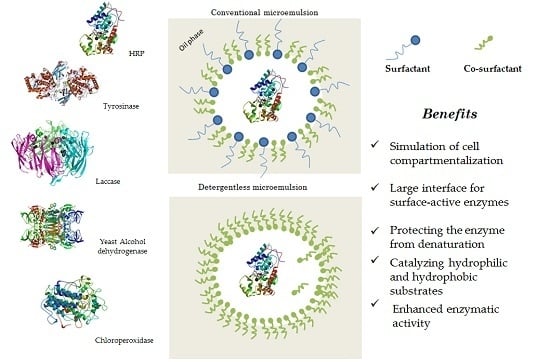
:1. Introduction
2. Oxidative Enzymes in Microemulsions
2.1. Heme Peroxidases
2.1.1. Horseradish Peroxidase
Effect of Surfactant Nature and Concentration
Effect of Co-Surfactant
Effect of the System’s Water Content
Effect of pH
2.1.2. Chloroperoxidases
2.1.3. Lignin Peroxidases (LiP)
2.2. Cholesterol Oxidase
2.3. Phenoloxidases
2.3.1. Laccases
2.3.2. Tyrosinase
2.4. Dehydrogenases
3. Superactivity
4. Surfactantless Microemulsions
5. Conclusion
Conflicts of Interest
References
- Bommarius, A.S.; Riebel, B.R. Biocatalysis, Fundamentals and Applications, 1st ed.; Wiley-VCH: Stuttgard, Germany, 2005; pp. 19–39. [Google Scholar]
- Dolman, D.; Newell, G.A.; Thurlow, M.D.; Dunford, B. A kinetic study of the reaction of horseradish peroxidase with hydrogen peroxide. Can. J. Biochem. 1975, 53, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Van Schijndel, J.W.P.M.; Barnett, P.; Roelse, J.; Vollenbroek, E.G.M.; Wever, R. The stability and steady-state kinetics of vanadium chloroperoxidase from the fungus Curvularia Inaequalis. Eur. J. Biochem. 1994, 225, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Zaks, A.; Klibanov, A.M. Enzyme-catalyzed processes in organic solvents. Proc. Natl. Acad. Sci. USA 1985, 82, 3192–3196. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Deetz, J.S.; Rozzell, J.D. Enzyme-catalysed reactions in non-aqueous media. 1988, 6, 7–9. [Google Scholar] [CrossRef]
- Kazandjian, R.Z.; Dordick, J.S.; Klibanov, A.M. Enzymatic analyses in organic solvents. Biotechnol. Bioeng. 1986, 28, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Danielsson, I.; Lindman, B. The definition of microemulsion. Colloids Surf. 1981, 3, 391–392. [Google Scholar] [CrossRef]
- Oldfield, C. Enzymes in water-in-oil microemulsions (‘Reversed micelles’): Principles and applications. Biotechnol. Genet. Eng. Rev. 1994, 12, 255–327. [Google Scholar] [CrossRef] [PubMed]
- Larsson, K.M.; Adlercreutz, P.; Mattiasson, B.; Olsson, U. Enzymatic catalysis in microemulsions: Enzyme reuse and product recovery. Biotechnol. Bioeng. 1990, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Regev, O.; Ezrahi, S.; Aserin, A.; Garti, N.; Wachtel, E.; Kaler, E.; Khan, A.; Talmon, Y. A study of the microstructure of a four-component nonionic microemulsion by cryo-TEM, NMR, SAXS, and SANS. Langmuir 1996, 12, 668–674. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papadimitriou, K.; Alexandraki, V.; Tsirvouli, E.; Chakim, Z.; Ghazal, A.; Mortensen, K.; Yaghmur, A.; Salentinig, S.; Papadimitriou, V.; et al. Microemulsions as potential carriers of nisin: Effect of composition on structure and efficacy. Langmuir 2016, 32, 8988–8998. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, P.D.I.; Robinson, B.H.; Freedman, R.B.; Oldfield, C. Activity of lipase in water-in-oil microemulsions. J. Chem. Soc. Faraday Trans. 1 1985, 81, 2667–2679. [Google Scholar] [CrossRef]
- Papadimitriou, V.; Sotiroudis, T.G.; Xenakis, A. Olive oil microemulsions: Enzymatic activities and structural characteristics. Langmuir 2007, 23, 2071–2077. [Google Scholar] [CrossRef] [PubMed]
- Avramiotis, S.; Papadimitriou, V.; Cazianis, C.T.; Xenakis, A. EPR studies of proteolytic enzymes in microemulsions. Colloids Surf. A 1998, 144, 295–304. [Google Scholar] [CrossRef]
- Khmelnitsky, Y.L.; Van Hoek, A.; Veeger, C.; Visser, A.J.W.G. Detergentless microemulsions as media for enzymatic reactions: Spectroscopic and ultracentrifugation studies. J. Phys. Chem. 1989, 93, 872–878. [Google Scholar] [CrossRef]
- Stamatis, H.; Xenakis, A.; Provelegiou, M.; Kolisis, F.N. Esterification reactions catalyzed by lipases in microemulsions: The role of enzyme localization in relation to its selectivity. Biotechnol. Bioeng. 1993, 42, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Misiorowski, R.L.; Wells, M. The activity of phospholipase A2 in reversed micelles of phosphatidylcholine in diethyl ether: Effect of water and cations. Biochemistry 1974, 13, 4921–4927. [Google Scholar] [CrossRef] [PubMed]
- Backlund, S.; Rantala, M.; Molander, O. Characterization of lecithin-based microemulsions used as media for a cholesterol oxidase-catalyzed reaction. Colloid Polym. Sci. 1994, 272, 1098–1103. [Google Scholar] [CrossRef]
- Gupta, S.; Mukhopadhyay, L.; Moulik, S.P. Kinetics in microemulsion V. Glucose oxidase catalyzed oxidation of β-d-glucose in aqueous, micellar and water-in-oil microemulsion media. Indian J. Biochem. Biophys. 2003, 40, 340–349. [Google Scholar] [PubMed]
- Stamatis, H.; Xenakis, A.; Kolisis, F.N. Bioorganic reactions in microemulsions: The case of lipases. Biotechnol. Adv. 1999, 17, 293–318. [Google Scholar] [CrossRef]
- Sanchez-Ferrer, A.; Perez-Gilabert, M.; Garcia-Carmona, F. Protein–interface interactions in reverse micelles. In Biocatalysis in Non-Conventional Media, Progress in Biotechnology; Tramper, J., Vermue, M.H., Beeftink, H.H., von Stockar, U., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 8, pp. 181–188. [Google Scholar]
- Avramiotis, S.; Cazianis, C.T.; Xenakis, A. Interfacial properties of lecithin microemulsions in the presence of lipase. A membrane spin-probe study. Langmuir 1999, 15, 2375–2379. [Google Scholar] [CrossRef]
- Xenakis, A.; Papadimitriou, V.; Stamatis, H.; Kolisis, F.N. Biocatalysis in microemulsions. In Microemulsions: Properties and Applications. Surfactant Science Series 144; CRC Press: Boca Raton, FL, USA, 2009; pp. 349–375. [Google Scholar]
- Lopez, F.; Cinelli, G.; Colella, M.; De Leonardis, A.; Palazzo, G.; Ambrosone, L. The role of microemulsions in lipase-catalyzed hydrolysis reactions. Biotechnol. Prog. 2014, 30, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Stamatis, H.; Xenakis, A.; Kolisis, F.N. Studies on enzyme reuse and product recovery in lipase-catalyzed reactions in microemulsions. Ann. N. Y. Acad. Sci. 1995, 750, 237–241. [Google Scholar] [CrossRef]
- Najjar, R.; Stubenrauch, C. Phase diagrams of microemulsions containing reducing agents and metal salts as bases for the synthesis of metallic nanoparticles. J. Colloid Interface Sci. 2009, 331, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.K.; Paul, B.K. Effect of NaCl and temperature on the water solubilization behavior of AOT/nonionics mixed reverse micellar systems stabilized in IPM oil. Colloids Surf. A 2005, 255, 165–180. [Google Scholar] [CrossRef]
- Hatzopoulos, M.H.; Eastoe, J.; Dowding, P.J.; Grillo, I. Cylinder to sphere transition in reverse microemulsions: The effect of hydrotropes. J. Colloid Interface Sci. 2013, 392, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Lopez, F.; Cinelli, G.; Ambrosone, L.; Colafemmina, G.; Ceglie, A.; Palazzo, G. Role of the cosurfactant in water-in-oil microemulsion: Interfacial properties tune the enzymatic activity of lipase. Colloids Surfaces A 2004, 237, 49–59. [Google Scholar] [CrossRef]
- Wong, M.; Thomas, J.K.; Nowak, T. Structure and state of water in reversed micelles. 3. J. Am. Chem. Soc. 1977, 99, 4730–4736. [Google Scholar] [CrossRef]
- El Seoud, O.A. Reverse Micelles; Luisa, P.L., Straud, B.E., Eds.; Plenum Press: New York, NY, USA, 1984; pp. 81–93. [Google Scholar]
- Zhou, N.; Li, Q.; Wu, J.; Chen, J.; Weng, S. Spectroscopic characterization of solubilized water in reversed micelles and microemulsions: Sodium bis(2-ethylhexyl) sulfosuccinate and sodium bis(2-ethylhexyl) phosphate in n-heptane. Langmuir 2001, 17, 4505–4509. [Google Scholar] [CrossRef]
- Ryu, K.; Dordick, J.S. How do organic solvents affect peroxidase structure and function? Biochemistry 1992, 31, 2588–2598. [Google Scholar] [CrossRef] [PubMed]
- Taurog, A.; Dorris, M.L.; Guziec, F.S. An unexpected side reaction in the guaiacol assay for peroxidase. Anal. Biochem. 1992, 205, 271–277. [Google Scholar] [CrossRef]
- Azevedo, A.M.; Martins, V.C.; Prazeres, D.M.F.; Vojinović, V.; Cabral, J.M.S.; Fonseca, L.P. Horseradish peroxidase: A valuable tool in biotechnology. Biotechnol. Annu. Rev. 2003, 9, 199–247. [Google Scholar] [PubMed]
- Cao, Q.; Quan, L.; He, C.; Li, N.; Li, K.; Liu, F. Partition of horseradish peroxidase with maintained activity in aqueous biphasic system based on ionic liquid. Talanta 2008, 77, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kadnikova, E.N.; Kostić, N.M. Oxidation of ABTS by hydrogen peroxide catalyzed by horseradish peroxidase encapsulated into sol-gel glass. Effects of glass matrix on reactivity. J. Mol. Catal. B 2002, 18, 39–48. [Google Scholar] [CrossRef]
- Sgalla, S.; Fabrizi, G.; Cacchi, S.; Macone, A.; Bonamore, A.; Boffi, A. Horseradish peroxidase in ionic liquids. Reactions with water insoluble phenolic substrates. J. Mol. Catal. B 2007, 44, 144–148. [Google Scholar] [CrossRef]
- Biswas, R.; Das, A.R.; Pradhan, T.; Touraud, D.; Kunz, W.; Mahiuddin, S. Spectroscopic studies of catanionic reverse microemulsion: Correlation with the superactivity of horseradish peroxidase enzyme in a restricted environment. J. Phys. Chem. B 2008, 112, 6620–6628. [Google Scholar] [CrossRef] [PubMed]
- Mahiuddin, S.; Renoncourt, A.; Bauduin, P.; Touraud, D.; Kunz, W. Horseradish peroxidase activity in a reverse catanionic microemulsion. Langmuir 2005, 21, 5259–5262. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, C.; Niu, J.; Li, S. FTIR study of horseradish peroxidase in reverse micelles. Biochem. Biophys. Res. Commun. 2001, 282, 1220–1223. [Google Scholar] [CrossRef] [PubMed]
- Bauduin, P.; Touraud, D.; Kunz, W.; Savelli, M.P.; Pulvin, S.; Ninham, B.W. The influence of structure and composition of a reverse SDS microemulsion on enzymatic activities and electrical conductivities. J. Colloid Interface Sci. 2005, 292, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Parida, S.; Parida, G.R.; Maitra, A.N. Studies on the catalytic activity of horseradish peroxidase hosted in Aerosol OT reverse micelles containing cholesterol. Colloids Surf. 1991, 55, 223–229. [Google Scholar] [CrossRef]
- Roy, S.; Dasgupta, A.; Das, P.K. Tailoring of horseradish peroxidase activity in cationic water-in-oil microemulsions. Langmuir 2006, 22, 4567–4573. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Das, D.; Das, P.K. Unsaturation at the surfactant head: Influence on the activity of lipase and horseradish peroxidase in reverse micelles. Biochem. Biophys. Res. Commun. 2007, 356, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Bauduin, P.; Nohmie, F.; Touraud, D.; Neueder, R.; Kunz, W.; Ninham, B.W. Hofmeister specific-ion effects on enzyme activity and buffer pH: Horseradish peroxidase in citrate buffer. J. Mol. Liq. 2006, 123, 14–19. [Google Scholar] [CrossRef]
- Gébicka, L.; Jurgas, M. Kinetics of horseradish peroxidase reactions in aqueous and reverse micelles composed of nonionic, polyoxyethylene alkyl ether surfactants. React. Kinet. Catal. Lett. 2004, 81, 291–296. [Google Scholar] [CrossRef]
- Motlekar, N.A.; Bhagwat, S.S. Activity of horseradish peroxidase in aqueous and reverse micelles and back-extraction from reverse-micellar phases. J. Chem. Technol. Biotechnol. 2001, 76, 643–649. [Google Scholar] [CrossRef]
- Kumar, A. pH dependent spectral characteristics of horseradish peroxidase dissolved in aqueous buffer as well as in nonionic reverse micellar systems. Ultra Chem. 2014, 10, 155–162. [Google Scholar]
- Shome, A.; Roy, S.; Das, P.K. Nonionic surfactants: A key to enhance the enzyme activity at cationic reverse micellar interface. Langmuir 2007, 23, 4130–4136. [Google Scholar] [CrossRef] [PubMed]
- Bera, A.; Ojha, K.; Kumar, T.; Mandal, A. Water solubilization capacity, interfacial compositions and thermodynamic parameters of anionic and cationic microemulsions. Colloids Surf. A 2012, 404, 70–77. [Google Scholar] [CrossRef]
- Digout, L.; Bren, K.; Palepu, R.; Moulik, S.P. Interfacial composition, structural parameters and thermodynamic properties of water-in-oil microemulsions. Colloid Polym. Sci. 2001, 279, 655–663. [Google Scholar] [CrossRef]
- Chern, C.S.; Wu, L.J. Kinetics of the microemulsion polymerization of styrene with short-chain alcohols as the cosurfactant. J. Polym. Sci. Part A 2001, 39, 898–912. [Google Scholar] [CrossRef]
- Hait, S.K.; Moulik, S.P. Interfacial composition and thermodynamics of formation of water/isopropyl myristate water-in-oil microemulsions stabilized by butan-1-ol and surfactants like cetyl pyridinium chloride, cetyl trimethyl ammonium bromide, and sodium dodecyl sulfate. Langmuir 2002, 18, 6736–6744. [Google Scholar] [CrossRef]
- Tonova, K.; Lazarova, Z. Reversed micelle solvents as tools of enzyme purification and enzyme-catalyzed conversion. Biotechnol. Adv. 2008, 26, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Kegel, W.K.; Overbeek, J.T.G.; Lekkerkerker, H.N.W. Thermodynamics of microemulsions I. In Handbook of Microemulsion Science and Technology, 1st ed.; Kumar, P., Mittal, K.L., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1999; pp. 13–44. [Google Scholar]
- Pietikainen, P.; Adlercreutz, P. Influence of the reaction medium on the product distribution of peroxidase-catalysed oxidation of p-cresol. Appl. Microbiol. Biotechnol. 1990, 33, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Martinek, K.; Levashov, A.V.; Klyachko, N.; Khmelnitski, Y.L.; Berezin, I.V. Micellar enzymology. Eur. J. Biochem. 1986, 155, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Tzika, E.D.; Christoforou, M.; Pispas, S.; Zervou, M.; Papadimitriou, V.; Sotiroudis, T.G.; Leontidis, E.; Xenakis, A. Influence of nanoreactor environment and substrate location on the activity of horseradish peroxidase in olive oil based water-in-oil microemulsions. Langmuir 2011, 27, 2692–2700. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, V.; Tzika, E.D.; Pispas, S.; Sotiroudis, T.G.; Xenakis, A. Microemulsions based on virgin olive oil: A model biomimetic system for studying native oxidative enzymatic activities. Colloids Surf. A 2011, 382, 232–237. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kamiya, N.; Goto, M. Biocatalysis in water-in-ionic liquid microemulsions: A case study with horseradish peroxidase. Langmuir 2009, 25, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.R.; Hager, L.P. Chloroperoxidase. I. Isolation and properties of the crystalline glycoprotein. J. Biol. Chem. 1966, 241, 1763–1768. [Google Scholar] [PubMed]
- Rai, G.P.; Sakai, S.; Florez, A.M.; Mogollon, L.; Hager, L.P. Directed evolution of chloroperoxidase for improved epoxidation and chlorination catalysis. Adv. Synth. Catal. 2001, 343, 638–645. [Google Scholar] [CrossRef]
- Franssen, M.C.R.; Weijnen, J.G.J.; Vincken, J.P.; Laane, C.; Van Der Plas, H.C. Chloroperoxidase-catalysed halogenation of apolar compounds using reversed micelles. Biocatal. Biotransformation 1988, 1, 205–216. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Han, B.; Li, J.; Li, Z.; Feng, X. Effect of compressed CO2 on the chloroperoxidase catalyzed halogenation of 1,3-dihydroxybenzene in reverse micelles. Phys. Chem. Chem. Phys. 2006, 8, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Ru, X.; Jiang, Y.; Hu, M.; Li, S.; Zhai, Q. Catalytic performance and thermostability of chloroperoxidase in reverse micelle: Achievement of a catalytically favorable enzyme conformation. J. Ind. Microbiol. Biotechnol. 2011, 38, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Samra, B.K.; Andersson, M.; Adlercreutz, P. Chloroperoxidase catalysed oxidation of benzyl alcohol using tert-butyl hydroperoxide oxidant in organic media. Biocatal. Biotransformation 1999, 17, 381–391. [Google Scholar] [CrossRef]
- Renirie, R.; Pierlot, C.; Wever, R.; Aubry, J.M. Singlet oxygenation in microemulsion catalysed by vanadium chloroperoxidase. J. Mol. Catal. B 2009, 56, 259–264. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin peroxidase of Phanerochaete cyrysosporium. Methods Enzymol. 1988, 161, 238–249. [Google Scholar]
- Kirk, T.K.; Farrell, R.L. Enzimatic “combustion”: The microbial degradation of lignin. Ann. Rev. Microbiol. 1987, 41, 465–505. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Michizoe, J.; Oakazaki, S.Y.; Furusaki, S.; Goto, M.; Tanaka, H.; Wariishi, H. Activation of lignin peroxidase in organic media by reversed micelles. Biotechnol. Bioeng. 2004, 88, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, X.R.; Huang, F.; Li, Y.Z.; Qu, Y.B.; Gao, P.J. Catalytic performance of lignin peroxidase in a novel reverse micelle. Colloids Surf. B 2008, 65, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.P.; Zhang, Y.; Huang, X.R.; Shi, C.H.; Liu, W.F.; Li, Y.Z.; Qu, Y.B.; Gao, P.J. Catalytic activities of fungal oxidases in hydrophobic ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate-based microemulsion. Colloids Surf. B 2008, 66, 146–149. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, J.; Wotherspoon, A.T.L.; Ansell, R.O.; Brooks, C.J.W. Cholesterol oxidase: Sources, physical properties and analytical applications. J. Steroid Biochem. Mol. Biol. 2000, 72, 169–195. [Google Scholar] [CrossRef]
- Lee, K.M.; Biellmann, J.-F. Cholesterol conversion to δ4-cholestenone by cholesterol oxidase in polyphasic systems: Extension to the selective oxidation of 7β-hydroxycholesterol. Tetrahedron 1988, 44, 1135–1139. [Google Scholar] [CrossRef]
- Laane, C.; Spruijt, R.; Hilhorst, R. Regulation and prediction of enzyme activity in reversed micelles. Biocatal. Biotransformation 1988, 1, 293–299. [Google Scholar] [CrossRef]
- Hedström, G.; Slotte, J.P.; Molander, O.; Rosenholm, J.B. Enzyme-catalyzed oxidation of cholesterol in physically characterized water-in-oil microemulsions. Biotechnol. Bioeng. 1992, 39, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Sánchez-Ferrer, A.; García-Carmona, F. Characterization of cholesterol oxidase activity in AOT-isooctane reverse micelles and its dependence on micelle size. Biotechnol. Lett. 1989, 11, 237–242. [Google Scholar] [CrossRef]
- Lee, K.M.; Biellmann, J.-F. Cholesterol oxidase in microemulsion: Enzymatic activity on a substrate of low water solubility and inactivation by hydrogen peroxide. Bioorg. Chem. 1986, 14, 262–273. [Google Scholar] [CrossRef]
- Gupte, A.; Nagarajan, R.; Kilara, A. Enzymic oxidation of cholesterol in reverse micelles. Ind. Eng. Chem. Res. 1995, 34, 2910–2922. [Google Scholar] [CrossRef]
- Gianfreda, L.; Xu, F.; Bollag, J.-M. Laccases: A useful group of oxidoreductive enzymes. Bioremediat. J. 1999, 3, 1–26. [Google Scholar] [CrossRef]
- Yaropolov, A.I.; Skorobogat’ko, O.V.; Vartanov, S.S.; Varfolomeyev, S.D. Laccase—Properties, catalytic mechanism, and applicability. Appl. Biochem. Biotechnol. 1994, 49, 257–280. [Google Scholar] [CrossRef]
- Karam, J.; Nicell, J.A. Potential applications of enzymes in waste treatment. J. Chem. Technol. Biotechnol. 1997, 69, 141–153. [Google Scholar] [CrossRef]
- Champagne, P.P.; Nesheim, M.E.; Ramsay, J.A. Effect of a non-ionic surfactant, Merpol, on dye decolorization of Reactive blue 19 by laccase. Enzyme Microb. Technol. 2010, 46, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Leontievsky, A.A.; Vares, T.; Lankinen, P.; Shergill, J.K.; Pozdnyakova, N.N.; Myasoedova, N.M.; Kalkkinen, N.; Golovleva, L.A.; Cammack, R.; Thurston, C.F.; et al. Blue and yellow laccases of ligninolytic fungi. FEMS Microbiol. Lett. 1997, 156, 9–14. [Google Scholar] [CrossRef]
- Rodakiewicz-Nowak, J.; Ito, M. Effect of AOT on enzymatic activity of the organic solvent resistant tyrosinase from Streptomyces sp. REN-21 in aqueous solutions and water-in-oil microemulsions. J. Colloid Interface Sci. 2005, 284, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Rodakiewicz-Nowak, J. Phenols oxidizing enzymes in water-restricted media. Top. Catal. 2000, 11, 419–434. [Google Scholar] [CrossRef]
- Okazaki, S.Y.; Michizoe, J.; Goto, M.; Furusaki, S.; Wariishi, H.; Tanaka, H. Oxidation of Bisphenol A catalyzed by laccase hosted in reversed micelles in organic media. Enzyme Microb. Technol. 2002, 31, 227–232. [Google Scholar] [CrossRef]
- Xue, L.; Qiu, H.; Li, Y.; Lu, L.; Huang, X.; Qu, Y. A novel water-in-ionic liquid microemulsion and its interfacial effect on the activity of laccase. Colloids Surf. B 2011, 82, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Sanchez-Ferrer, A.; García-Carmona, F. Characteristics of tyrosinase in AOT-lsooctane reverse micelles. Biotechnol. Bioeng. 1989, 34, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Rojo, M.; Gόmez, M.; Estrada, P. Polyphenol oxidase in reverse micelles of AOT/cyclohexane: A thermostability study. J. Chem. Technol. Biotechnol. 2001, 77, 69–77. [Google Scholar] [CrossRef]
- Shipovskov, S.; Ferapontova, E.; Ruzgas, T.; Levashov, A. Stabilisation of tyrosinase by reversed micelles for bioelectrocatalysis in dry organic media. Biochim. Biophys. Acta 2003, 1620, 119–124. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Bru, R.; García-Carmona, F. Kinetic properties of polyphenoloxidase in organic solvents A study in Brij 96-cyclohexane reverse micelles. FEBS Lett. 1988, 233, 363–366. [Google Scholar] [CrossRef]
- Yang, Z.; Robb, D.A. Tyrosinase activity in reversed micelles. Biocatal. Biotransformation 2005, 23, 423–430. [Google Scholar] [CrossRef]
- Yang, Z.; Robb, D.A. Comparison of tyrosinase activity and stability in aqueous and nearly nonaqueous environments. Enzym. Microb. Technol. 1993, 15, 1030–1036. [Google Scholar] [CrossRef]
- Shipovskov, S.; Levashov, A. Entrapping of tyrosinase in a system of reverse micelles. Biocatal. Biotransformation 2004, 22, 57–60. [Google Scholar] [CrossRef]
- Papadimitriou, V.; Sotiroudis, T.G.; Xenakis, A. Olive oil microemulsions as a biomimetic medium for enzymatic studies: Oxidation of oleuropein. J. Am. Oil Chem. Soc. 2005, 82, 335–340. [Google Scholar] [CrossRef]
- Tzika, E.D.; Papadimitriou, V.; Sotiroudis, T.G.; Xenakis, A. Oxidation of oleuropein studied by EPR and spectrophotometry. Eur. J. Lipid Sci. Technol. 2008, 110, 149–157. [Google Scholar] [CrossRef]
- De Smidt, O.; Du Preez, J.C.; Albertyn, J. The alcohol dehydrogenases of Saccharomyces cerevisiae: A comprehensive review. FEMS Yeast Res. 2008, 8, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Luisi, P.L. Micellar solubilization of biopolymers in hydrocarbon solvents. II. The case of horse liver alcohol dehydrogenase. J. Solid-Phase Biochem. 1980, 5, 269–282. [Google Scholar] [CrossRef]
- Martinek, K.; Levashov, A.V.; Khmelnitsky, Y.L.; Klyachko, N.L.; Berezin, I.V. Colloidal solution of water in organic solvents: a microheterogeneous medium for enzymatic reactions. Science 1982, 218, 889–891. [Google Scholar] [CrossRef] [PubMed]
- Samama, J.-P.; Lee, K.M.; Biellmann, J.-F. Enzymes and microemulsions. Activity and kinetic properties of liver alcohol dehydrogenase in ionic water-in-oil microemulsions. Eur. J. Biochem. 1987, 163, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Berezin, I.V.; Martinek, K. Catalysis by enzymes entrapped in reversed micelles of surfactants in organic solvents. Ann. N. Y. Acad. Sci. 1984, 434, 577–579. [Google Scholar] [CrossRef]
- Larsson, K.M.; Adlerccreutz, P.; Mattiasson, B. Activity and stability of horse-liver alcohol dehydrogenase in sodium dioctylsulfosuccinate/cyclohexane reverse micelles. Eur. J. Biochem. 1987, 166, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Vos, K.; Laane, C.; Vanhoek, A.; Veeger, C.; Visser, A.J.W.G. Spectroscopic properties of horse liver alcohol-dehydrogenase in reversed micellar solutions. Eur. J. Biochem. 1987, 169, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Strambini, G.B.; Gonnelli, M. Protein dynamical structure by tryptophan phosphorescence and enzymatic activity in reverse mlcelles: 2. Alkaline phosphatase. J. Phys. Chem. 1988, 92, 2584–2587. [Google Scholar] [CrossRef]
- Lee, K.M.; Biellmann, J.F. Enzyme and organic solvents: Horse liver alcohol dehydrogenase in non-ionic microemulsion: Stability and activity. FEBS Lett. 1987, 223, 33–36. [Google Scholar] [CrossRef]
- Sarcar, S.; Jain, T.K.; Maitra, A. Activity and stability of yeast alcohol dehydrogenase (YADH) entrapped in aerosol OT reverse micelles. Biotechnol. Bioeng. 1992, 39, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mozumdar, S.; Maitra, A. Activity and conformation of yeast alcohol dehydrogenase (YADH) entrapped in reverse micelles. J. Colloid Interface Sci. 2000, 230, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.H.; Liao, M.-H. Effects of mixed reverse micellar structure on stability and activity of yeast alcohol dehydrogenase. J. Mol. Catal. B 2002, 18, 155–162. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, X.; Li, Y. Negative effect of [bmim][PF6] on the catalytic activity of alcohol dehydrogenase: Mechanism and prevention. J. Chem. Technol. Biotechnol. 2007, 82, 1115–1121. [Google Scholar] [CrossRef]
- Sintra, T.E.; Ventura, S.P.M.; Coutinho, J.A.P. Superactivity induced by micellar systems as the key for boosting the yield of enzymatic reactions. J. Mol. Catal. B 2014, 107, 140–151. [Google Scholar] [CrossRef]
- Schirmer, C.; Liu, Y.; Touraud, D.; Meziani, A.; Pulvin, S.; Kunz, W. Horse liver alcohol dehydrogenase as a probe for nanostructuring effects of alcohols in water/nonionic surfactant systems. J. Phys. Chem. B 2002, 106, 7414–7421. [Google Scholar] [CrossRef]
- Elhefnawy, M.E. The influence of composition and structure of water-in-oil microemulsions on activities of Iraqi Turnip peroxidase. Arab. J. Chem. 2012, 5, 73–76. [Google Scholar] [CrossRef]
- Zemb, T.N.; Klossek, M.; Lopian, T.; Marcus, J.; Schöettl, S.; Horinek, D.; Prevost, S.F.; Touraud, D.; Diat, O.; Marčelja, S.; et al. How to explain microemulsions formed by solvent mixtures without conventional surfactants. Proc. Natl. Acad. Sci. USA 2016, 113, 4260–4265. [Google Scholar] [CrossRef] [PubMed]
- Diat, O.; Klossek, M.L.; Touraud, D.; Deme, B.; Grillo, I.; Kunz, W.; Zemb, T. Octanol-rich and water-rich domains in dynamic equilibrium in the pre-ouzo region of ternary systems containing a hydrotrope. J. Appl. Crystallogr. 2013, 46, 1665–1669. [Google Scholar] [CrossRef]
- Khmelnitsky, Y.L.; Hilhorst, R.; Verger, C. Detergentless microemulsions as media for enzymatic reactions: Cholesterol oxidation catalyzed by cholesterol oxidase. Eur. J. Biochem. 1988, 176, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Friberg, S.E.; Aikens, P.A. A phase diagram approach to microemulsions. In Microemulsions: Properties and Applications; Fanun, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2009; Volume 144, pp. 2–15. [Google Scholar]
- Smith, G.D.; Donelan, C.E.; Barden, R.E. Oil-continuous microemulsions composed of hexane, water, and 2-propanol. J. Colloid Interface Sci. 1977, 60, 488–496. [Google Scholar] [CrossRef]
- Khmelnitsky, I.; Zharinova, I.N.; Berezin, I.V.; Levashov, A.V.; Martinek, K. Detergentless microemulsions—A new microheterogeneous medium for enzymatic-reactions. Dokl. Akad. Nauk SSSR 1986, 289, 1178–1181. [Google Scholar]
- Zoumpanioti, M.; Karali, M.; Xenakis, A.; Stamatis, H. Lipase biocatalytic processes in surfactant free microemulsion-like ternary systems and related organogels. Enzym. Microb. Technol. 2006, 39, 531–539. [Google Scholar] [CrossRef]
- Khmelnitsky, Y.L.; Gladilin, A.K.; Neverova, I.N.; Levashov, A.V.; Martinek, K. Detergentless microemulsions as media for enzymatic reactions: Catalytic properties of laccase in the ternary system hexane-2-propanol-water. Collect. Czechoslov. Chem. Commun. 1990, 55, 555–563. [Google Scholar] [CrossRef]
- Xenakis, A.; Zoumpanioti, M.; Stamatis, H. Enzymatic reactions in structured surfactant-free microemulsions. Curr. Opin. Colloid Interface Sci. 2016, 22, 41–45. [Google Scholar] [CrossRef]
- Borys, N.F.; Holt, S.L.; Barden, R.E. Detergentless water/oil microemulsions. III. Effect of KOH on phase diagram and effect of solvent composition on base hydrolysis of esters. J. Colloid Interface Sci. 1979, 71, 526–532. [Google Scholar] [CrossRef]
- Vulfson, E.N.; Ahmed, G.; Gill, I.; Kozlov, I.A.; Goodenough, P.W.; Law, B.A. Alterations to the catalytic properties of polyphenoloxidase in detergentless microemulsions and ternary water-organic solvent mixtures. Biotechnol. Lett. 1991, 13, 91–96. [Google Scholar] [CrossRef]
- Zoumpanioti, M.; Stamatis, H.; Papadimitriou, V.; Xenakis, A. Spectroscopic and catalytic studies of lipases in ternary hexane-1-propanol-water surfactantless microemulsion systems. Colloids Surf. B 2006, 47, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lund, G.; Holt, S.L. Detergentless water/oil microemulsions: IV. The ternary pseudo-phase diagram for and properties of the system toluene/2-propanol/water. J. Am. Oil Chem. Soc. 1980, 57, 264–267. [Google Scholar] [CrossRef]
- Roux-Desgranges, G.; Grolier, J.P.E.; Villamañan, M.A.; Casanova, C. Role of alcohol in microemulsions. III. Volumes and heat capacities in the continuous phase water-n-butanol-toluene of reverse micelles. Fluid Phase Equilib. 1986, 25, 209–230. [Google Scholar] [CrossRef]
- Tzialla, A.A.; Kalogeris, E.; Gournis, D.; Sanakis, Y.; Stamatis, H. Enhanced catalytic performance and stability of chloroperoxidase from Caldariomyces fumago in surfactant free ternary water-organic solvent systems. J. Mol. Catal. B 2008. [Google Scholar] [CrossRef]
- Tzialla, A.A.; Taha, A.A.; Kalogeris, E.; Stamatis, H. Improving the catalytic performance of fungal laccases in monoterpene-based reaction systems. Biotechnol. Lett. 2009, 31, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Fedorak, P.M.; Semple, K.M.; Vazquez-Duhalt, R.; Westlake, D.W.S. Chloroperoxidase-mediated modifications of petroporphyrins and asphaltenes. Enzym. Microb. Technol. 1993, 15, 429–437. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsou, E.; Xenakis, A.; Zoumpanioti, M. Oxidation Catalysis by Enzymes in Microemulsions. Catalysts 2017, 7, 52. https://doi.org/10.3390/catal7020052
Mitsou E, Xenakis A, Zoumpanioti M. Oxidation Catalysis by Enzymes in Microemulsions. Catalysts. 2017; 7(2):52. https://doi.org/10.3390/catal7020052
Chicago/Turabian StyleMitsou, Evgenia, Aristotelis Xenakis, and Maria Zoumpanioti. 2017. "Oxidation Catalysis by Enzymes in Microemulsions" Catalysts 7, no. 2: 52. https://doi.org/10.3390/catal7020052
APA StyleMitsou, E., Xenakis, A., & Zoumpanioti, M. (2017). Oxidation Catalysis by Enzymes in Microemulsions. Catalysts, 7(2), 52. https://doi.org/10.3390/catal7020052