Hydrothermal Fabrication of High Specific Surface Area Mesoporous MgO with Excellent CO2 Adsorption Potential at Intermediate Temperatures
Abstract
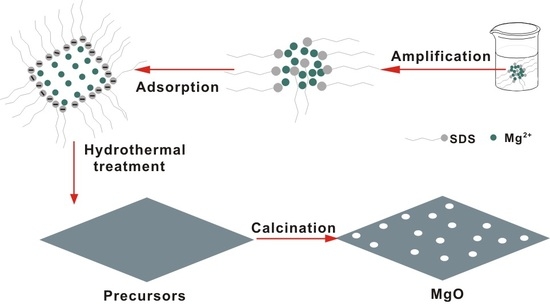
:1. Introduction
2. Results and Discussion
2.1. Evaluation of CO2 Adsorption Capacity
2.1.1. The Influence of Different Types of Surfactant on CO2 Capture
2.1.2. The Influence of the Ratio of Ethanol to Water on CO2 Capture
2.1.3. The Influence of the SDS Amount on CO2 Capture
2.1.4. The Influence of Reaction Time on CO2 Capture
2.1.5. The Influence of the Reaction Temperature on CO2 Capture
2.2. Characterization of SDS-Assisted MgO
2.3. Cycling Stability Tests
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Sample Characterization
3.4. Evaluation of CO2 Adsorption Capacity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Markewitz, P.; Kuckshinrichs, W.; Leitner, W.; Linssen, J.; Zapp, P.; Bongartz, R.; Schreiber, A.; Müller, T.E. Worldwide innovations in the development of carbon capture technologies and the utilization of CO2. Energy Environ. Sci. 2012, 5, 7281–7305. [Google Scholar] [CrossRef]
- Mikkelsen, M.; Jørgensen, M.; Krebs, F.C. The teraton challenge. A review of fixation and transformation of carbon dioxide. Energy Environ. Sci. 2010, 3, 43–81. [Google Scholar] [CrossRef]
- Schrag, D.P. Preparing to capture CO2. Science 2007, 315, 812–813. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 capture by solid adsorbents and their applications: Current status and new trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.S.; Duan, Y.; King, D.L.; Singh, P.; Li, L. Roles of double salt formation and NaNO3 in Na2CO3-promoted MgO absorbent for intermediate temperature CO2 removal. Int. J. Greenh. Gas Control 2013, 12, 351–358. [Google Scholar] [CrossRef]
- Chakradhar, A.; Burghaus, U. Carbon dioxide adsorption on MgO(001)—CO2 kinetics and dynamics. Surf. Sci. 2013, 616, 171–177. [Google Scholar] [CrossRef]
- Plaza, M.G.; Pevida, C.; Arias, B.; Casal, M.D.; Martín, C.F.; Fermoso, J.; Rubiera, F.; Pis, J.J. Different approaches for the development of low-cost CO2 adsorbents. J. Environ. Eng. 2009, 135, 426–432. [Google Scholar] [CrossRef]
- Harada, T.; Simeon, F.; Hamad, E.Z.; Hatton, T.A. Alkali metal nitrate-promoted high-capacity MgO adsorbents for regenerable CO2 capture at moderate temperatures. Chem. Mater. 2015, 27, 1943–1949. [Google Scholar] [CrossRef]
- Goff, G.S.; Rochelle, G.T. Monoethanolamine degradation: O2 mass transfer effects under CO2 capture conditions. Ind. Eng. Chem. Res. 2004, 43, 6400–6408. [Google Scholar] [CrossRef]
- Vu, A.-T.; Park, Y.; Jeon, P.R.; Lee, C.-H. Mesoporous MgO sorbent promoted with KNO3 for CO2 capture at intermediate temperatures. Chem. Eng. J. 2014, 258, 254–264. [Google Scholar] [CrossRef]
- Vu, A.-T.; Ho, K.; Jin, S.; Lee, C.-H. Double sodium salt-promoted mesoporous MgO sorbent with high CO2 sorption capacity at intermediate temperatures under dry and wet conditions. Chem. Eng. J. 2016, 291, 161–173. [Google Scholar] [CrossRef]
- Lepaumier, H.; Martin, S.; Picq, D.; Delfort, B.; Carrette, P.-L. New amines for CO2 capture. III. effect of alkyl chain length between amine functions on polyamines degradation. Ind. Eng. Chem. Res. 2010, 49, 4553–4560. [Google Scholar] [CrossRef]
- Knuutila, H.; Svendsen, H.; Anttila, M. CO2 capture from coal-fired power plants based on sodium carbonate slurry; a systems feasibility and sensitivity study. Int. J. Greenh. Gas Control 2009, 3, 143–151. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O’Hare, D.; Wang, Q. Comprehensive investigation of CO2 adsorption on Mg-Al-CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A 2013, 1, 12782–12790. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.; Rodrigues, A.E. Adsorption of carbon dioxide at high temperature—A review. Sep. Purif. Technol. 2002, 26, 195–205. [Google Scholar] [CrossRef]
- Yong, Z.; Mata, V.; Rodrigues, A.E. Adsorption of carbon dioxide onto hydrotalcite-like compounds (HTlcs) at high temperatures. Ind. Eng. Chem. Res. 2001, 40, 204–209. [Google Scholar] [CrossRef]
- Lee, J.M.; Min, Y.J.; Lee, K.B.; Jeon, S.G.; Na, J.G.; Ryu, H.J. Enhancement of CO2 sorption uptake on hydrotalcite by impregnation with K2CO3. Langmuir 2010, 26, 18788–18797. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Alpay, E. Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem. Eng. Sci. 2000, 55, 3461–3474. [Google Scholar] [CrossRef]
- Bian, S.-W.; Baltrusaitis, J.; Galhotra, P.; Grassian, V.H. A template-free, thermal decomposition method to synthesize mesoporous MgO with a nanocrystalline framework and its application in carbon dioxide adsorption. J. Mater. Chem. 2010, 20, 8705–8710. [Google Scholar] [CrossRef]
- Hanif, A.; Dasgupta, S.; Nanoti, A. Facile synthesis of high surface area mesoporous MgO with excellent high temperature CO2 adsorption potential. Ind. Eng. Chem. Res. 2016, 55, 8070–8078. [Google Scholar] [CrossRef]
- Zhao, Z.; Dai, H.; Du, Y.; Deng, J.; Zhang, L.; Shi, F. Solvo- or hydrothermal fabrication and excellent carbon dioxide adsorption behaviors of magnesium oxides with multiple morphologies and porous structures. Mater. Chem. Phys. 2011, 128, 348–356. [Google Scholar] [CrossRef]
- Han, K.K.; Zhou, Y.; Lin, W.G.; Zhu, J.H. One-pot synthesis of foam-like magnesia and its performance in CO2 adsorption. Microporous Mesoporous Mater. 2013, 169, 112–119. [Google Scholar] [CrossRef]
- Seo, Y.; Jo, S.H.; Ryu, C.K.; Yi, C.K. Effects of water vapor pretreatment time and reaction temperature on CO2 capture characteristics of a sodium-based solid sorbent in a bubbling fluidized-bed reactor. Chemosphere 2007, 69, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, L.; Xiao, Y. Affecting mechanism of activation conditions on the performance of NaNO3-modified dolomite for CO2 capture. Asia-Pac. J. Chem. Eng. 2015, 10, 754–763. [Google Scholar] [CrossRef]
- Harada, T.; Hatton, T.A. Colloidal nanoclusters of MgO coated with alkali metal nitrates/nitrites for rapid high capacity CO2 capture at moderate temperature. Chem. Mater. 2015, 27, 8153–8161. [Google Scholar] [CrossRef]
- Hanif, A.; Dasgupta, S.; Nanoti, A. High temperature CO2 adsorption by mesoporous silica supported magnesium aluminum mixed oxide. Chem. Eng. J. 2015, 280, 703–710. [Google Scholar] [CrossRef]
- Zhang, K.; Li, X.S.; Chen, H.; Singh, P.; King, D.L. Molten salt promoting effect in double salt CO2 absorbents. J. Phys. Chem. C 2016, 120, 1089–1096. [Google Scholar] [CrossRef]
- Ding, Y.; Alpay, E. High temperature recovery of CO2 from flue gases using hydrotalcite adsorbent. Process Saf. Environ. Prot. 2001, 79, 45–51. [Google Scholar] [CrossRef]
- Ram Reddy, M.K.; Xu, Z.P.; Lu, G.Q.; Diniz da Costa, J.C. Layered double hydroxides for CO2 capture: Structure evolution and regeneration. Ind. Eng. Chem. Res. 2006, 45, 7504–7509. [Google Scholar] [CrossRef]
- Yavuz, C.T.; Shinall, B.D.; Iretskii, A.V.; White, M.G.; Golden, T.; Atilhan, M.; Ford, P.C.; Stucky, G.D. Markedly improved CO2 capture efficiency and stability of gallium substituted hydrotalcites at elevated temperatures. Chem. Mater. 2009, 21, 3473–3475. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and trends in CO2 capture/separation technologies: A review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Song, G.; Zhu, X.; Chen, R.; Liao, Q.; Ding, Y.-D.; Chen, L. An investigation of CO2 adsorption kinetics on porous magnesium oxide. Chem. Eng. J. 2016, 283, 175–183. [Google Scholar] [CrossRef]
- Han, K.K.; Zhou, Y.; Chun, Y.; Zhu, J.H. Efficient MgO-based mesoporous CO2 trapper and its performance at high temperature. J. Hazard. Mater. 2012, 203, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Siriwardane, R.V.; Robert, W.; Stevens, J. Novel regenerable magnesium hydroxidesorbents for CO2 capture at warm gas temperatures. Ind. Eng. Chem. Res. 2009, 48, 2135–2141. [Google Scholar] [CrossRef]
- Choi, D.-H.; Lee, J.B.; Eom, T.H.; Baek, J.I.; Jegarl, S.; Ryu, C.K. Study of MgO-based dry regenerable sorbent for sorption enhanced water gas shift reaction. Renew. Energy 2013, 54, 144–149. [Google Scholar] [CrossRef]
- Henrist, C.; Mathieu, J.P.; Vogels, C.; Rulmont, A.; Cloots, R.J. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef]
- Ruminski, A.M.; Jeon, K.-J.; Urban, J.J. Size-dependent CO2 capture in chemically synthesized magnesium oxide nanocrystals. J. Mater. Chem. 2011, 21, 11486–11491. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, G.; Wu, H.; Hai, B.; Wang, L.; Qian, Y. Nanoscale magnesium hydroxide and magnesium oxide powders: Control over size, shape, and structure via hydrothermal synthesis. Chem. Mater. 2001, 13, 435–440. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croue, J.P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef] [PubMed]
- Kruk, M.; Jaroniec, M. Gas adsorption characterization of ordered organic-inorganic nanocomposite materials. Chem. Mater. 2001, 13, 3169–3183. [Google Scholar] [CrossRef]
- Dhaouadi, H.; Chaabane, H.; Touati, F. Mg(OH)2 nanorods synthesized by a facile hydrothermal method in the presence of CTAB. Nano-Micro Lett. 2011, 3, 153–159. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, J.; Zhang, Y.; Gao, W.; Harada, T.; Huang, L.; Hatton, T.A.; Wang, Q. Alkali nitrates molten salt modified commercial MgO for intermediate-temperature CO2 capture: Optimization of the Li/Na/K ratio. Ind. Eng. Chem. Res. 2017, 56, 1509–1517. [Google Scholar] [CrossRef]
- Sutradhar, N.; Sinhamahapatra, A.; Pahari, S.K.; Pal, P.; Bajaj, H.C.; Mukhopadhyay, I.; Panda, A.B. Controlled synthesis of different morphologies of MgO and their use as solid base catalysts. J. Phys. Chem. C 2011, 115, 12308–12316. [Google Scholar] [CrossRef]
- Faria, M.; de Pinho, M.N. Phase segregation and gas permeation properties of poly(urethane urea) bi-soft segment membranes. Eur. Polym. J. 2016, 82, 260–276. [Google Scholar] [CrossRef]
- Ren, L.-F.; Geng, J.; Chen, T.; Guo, P.-Y.; Qiang, T.-T. Synthesis and application of hyperbranched poly(urethane-urea) finishing agent with amino groups. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Abbasi, E.; Hassanzadeh, A.; Abbasian, J. Regenerable MgO-based sorbent for high temperature CO2 removal from syngas: 2. Two-zone variable diffusivity shrinking core model with expanding product layer. Fuel 2013, 105, 128–134. [Google Scholar] [CrossRef]
- Abbasi, E.; Hassanzadeh, A.; Zarghami, S.; Arastoopour, H.; Abbasian, J. Regenerable MgO-based sorbent for high temperature CO2 removal from syngas: 3. CO2 capture and sorbent enhanced water gas shift reaction. Fuel 2013, 137, 260–268. [Google Scholar] [CrossRef]










| Sample | Surfactant | Ethanol/Water Ratio | Hydrothermal Conditions (°C and h) | Calcination Conditions (°C and h) | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | Crystalline Size (nm) | CO2 Uptake (mmol g−1) |
|---|---|---|---|---|---|---|---|---|---|
| MgO | Surfactant-free | 1:1 | 120 and 12 | 400 and 5 | 194.3 | 0.30 | 3.05 | 1.3 | 0.86 |
| MgO | P-123 | 1:1 | 120 and 12 | 400 and 5 | 305.7 | 0.42 | 2.78 | 2.1 | 0.78 |
| MgO | PVP | 1:1 | 120 and 12 | 400 and 5 | 302.3 | 0.36 | 2.40 | 2.0 | 0.86 |
| MgO | CTAB | 1:1 | 120 and 12 | 400 and 5 | 341.4 | 0.49 | 2.90 | 3.2 | 0.91 |
| MgO | SDS | 1:1 | 120 and 12 | 400 and 5 | 321.7 | 0.40 | 2.51 | 7.1 | 0.92 |
| MgO-TD | - | 1:1 | - | 400 and 5 | 363.4 | 0.53 | 2.91 | 6.0 | 0.29 |
| Light MgO | - | 1:1 | - | - | 25.1 | 0.17 | 13.22 | 32.4 | 0.05 |
| Sample | Ethanol/Water Ratio | Hydrothermal Conditions (°C and h) | Calcination Conditions (°C and h) | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | Crystalline Size (nm) | CO2 Uptake (mmol g−1) |
|---|---|---|---|---|---|---|---|---|
| MgO-SDS | 100% ethanol | 120 and 12 | 400 and 5 | 58.7 | 0.69 | 23.52 | 18.6 | 0.04 |
| MgO-SDS | 40:1 | 120 and 12 | 400 and 5 | 185.8 | 0.81 | 8.67 | 5.3 | 0.36 |
| MgO-SDS | 10:1 | 120 and 12 | 400 and 5 | 272.4 | 0.64 | 4.73 | 2.8 | 0.44 |
| MgO-SDS | 100% water | 120 and 12 | 400 and 5 | 321.3 | 0.30 | 1.88 | 5.4 | 0.96 |
| Sample | Ethanol/Water Ratio | Loading (g) | Hydrothermal Conditions (°C and h) | Calcination Conditions (°C and h) | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | Crystalline Size (nm) | CO2 Uptake (mmol g−1) |
|---|---|---|---|---|---|---|---|---|---|
| MgO-SDS | 100% water | 0.08 | 120 and 12 | 400 and 5 | 348.3 | 0.36 | 2.04 | 4.9 | 0.85 |
| MgO-SDS | 100% water | 0.1 | 120 and 12 | 400 and 5 | 321.3 | 0.30 | 1.88 | 5.4 | 0.96 |
| MgO-SDS | 100% water | 0.5 | 120 and 12 | 400 and 5 | 501.3 | 0.64 | 2.55 | 2.1 | 0.93 |
| MgO-SDS | 100% water | 1.0 | 120 and 12 | 400 and 5 | 413.4 | 0.39 | 2.31 | 10.0 | 0.90 |
| Sample | Ethanol/Water Ratio | Loading (g) | Hydrothermal Conditions (°C and h) | Calcination Conditions (°C and h) | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | Crystalline Size (nm) | CO2 Uptake (mmol g−1) |
|---|---|---|---|---|---|---|---|---|---|
| MgO-SDS | 100% water | 0.1 | 120 and 6 | 400 and 5 | 369.1 | 0.35 | 1.90 | 11.6 | 0.94 |
| MgO-SDS | 100% water | 0.1 | 120 and 12 | 400 and 5 | 321.3 | 0.30 | 1.88 | 5.4 | 0.96 |
| MgO-SDS | 100% water | 0.1 | 120 and 18 | 400 and 5 | 276.6 | 0.40 | 2.19 | 20.3 | 0.83 |
| MgO-SDS | 100% water | 0.1 | 120 and 48 | 400 and 5 | 204.0 | 0.19 | 1.86 | 55.0 | 0.79 |
| MgO-SDS | 100% water | 0.1 | 120 and 72 | 400 and 5 | 375.3 | 0.34 | 1.82 | 43.3 | 0.55 |
| Sample | Ethanol/Water Ratio | Loading (g) | Hydrothermal Conditions (°C and h) | Calcination Conditions (°C and h) | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Average Pore Size (nm) | Crystalline Size (nm) | CO2 Uptake (mmol g−1) |
|---|---|---|---|---|---|---|---|---|---|
| MgO-SDS | 100% water | 0.1 | 100 and 12 | 400 and 5 | 106.8 | 0.50 | 9.44 | 63.4 | 0.45 |
| MgO-SDS | 100% water | 0.1 | 120 and 12 | 400 and 5 | 321.3 | 0.30 | 1.88 | 5.4 | 0.96 |
| MgO-SDS | 100% water | 0.1 | 140 and 12 | 400 and 5 | 181.7 | 0.22 | 2.40 | 50.1 | 0.69 |
| MgO-SDS | 100% water | 0.1 | 160 and 12 | 400 and 5 | 41.1 | 0.07 | 3.49 | 50.7 | 0.15 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Zhou, T.; Louis, B.; Wang, Q. Hydrothermal Fabrication of High Specific Surface Area Mesoporous MgO with Excellent CO2 Adsorption Potential at Intermediate Temperatures. Catalysts 2017, 7, 116. https://doi.org/10.3390/catal7040116
Gao W, Zhou T, Louis B, Wang Q. Hydrothermal Fabrication of High Specific Surface Area Mesoporous MgO with Excellent CO2 Adsorption Potential at Intermediate Temperatures. Catalysts. 2017; 7(4):116. https://doi.org/10.3390/catal7040116
Chicago/Turabian StyleGao, Wanlin, Tuantuan Zhou, Benoit Louis, and Qiang Wang. 2017. "Hydrothermal Fabrication of High Specific Surface Area Mesoporous MgO with Excellent CO2 Adsorption Potential at Intermediate Temperatures" Catalysts 7, no. 4: 116. https://doi.org/10.3390/catal7040116
APA StyleGao, W., Zhou, T., Louis, B., & Wang, Q. (2017). Hydrothermal Fabrication of High Specific Surface Area Mesoporous MgO with Excellent CO2 Adsorption Potential at Intermediate Temperatures. Catalysts, 7(4), 116. https://doi.org/10.3390/catal7040116