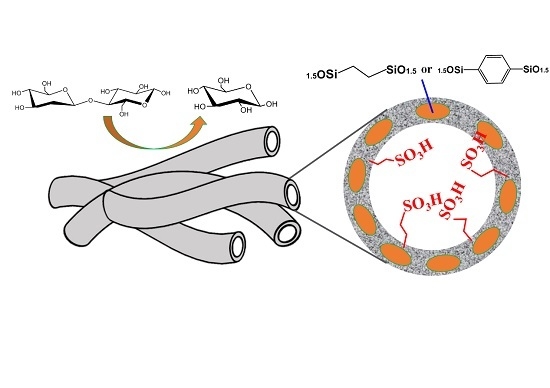
Well-Shaped Sulfonic Organosilica Nanotubes with High Activity for Hydrolysis of Cellobiose
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Sulfonic Organosilica Nanotubes
2.2. Hydrolysis of Cellobiose
3. Materials and Methods
3.1. Chemicals and Regents
3.2. Catalyst Preparation
3.2.1. Synthesis of Sulfonic Ethyl-Bridged Organosilica Nanotubes
3.2.2. Synthesis of Sulfonic Phenyl-Bridged Organosilica Nanotubes
3.2.3. Synthesis of Sulfonic Silica Nanotubes
3.3. Characterization
3.4. Catalytic Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-B.; Fu, Y. Hydrolysis of cellulose to glucose by solid acid catalysts. Green Chem. 2013, 15, 1095–1111. [Google Scholar] [CrossRef]
- Salesch, T.; Bachmann, S.; Brugger, S.; Rabelo-Schaefer, R.; Albert, K.; Steinbrecher, S.; Plies, E.; Mehdi, A.; Reyé, C.; Corriu, R.J.P.; et al. New inorganic ± organic hybrid materials for HPLC separationobtained by direct synthesis in the presence of a surfactant. Adv. Funct. Mater. 2002, 12, 134–142. [Google Scholar] [CrossRef]
- Melero, J.A.; van Grieken, R.; Morales, G. Advances in the synthesis and catalytic applications of organosulfonic-functionalized mesostructured materials. Chem. Rev. 2006, 106, 3790–3812. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Iborra, S.; Velty, A. Chemical routes for the transformation of biomass into chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Van der Voort, P.; Esquivel, D.; de Canck, E.; Goethals, F.; van Driessche, I.; Romero-Salguero, F.J. Periodic mesoporous organosilicas: From simple to complex bridges; a comprehensive overview of functions, morphologies and applications. Chem. Soc. Rev. 2013, 42, 3913–3955. [Google Scholar] [CrossRef] [PubMed]
- López, M.I.; Esquivel, D.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J.; van der Voort, P. A “one-step” sulfonic acid PMO as a recyclable acid catalyst. J. Catal. 2015, 326, 139–148. [Google Scholar] [CrossRef]
- Zhang, X.; Su, F.; Song, D.; An, S.; Lu, B.; Guo, Y. Preparation of efficient and recoverable organosulfonic acid functionalized alkyl-bridged organosilica nanotubes for esterification and transesterification. Appl. Catal. B Environ. 2015, 163, 50–62. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Guan, Z.; Liu, J.; Zhao, J.; Yang, Y.; Yang, Q. Organosilica nanotubes: Large-scale synthesis and encapsulation of metal nanoparticles. Chem. Commun. Camb. 2011, 47, 8073–8075. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; An, S.; Sun, Y.; Zhang, P.; Guo, Y.; Zhou, D. Ethane-bridged organosilica nanotubes functionalized with arenesulfonic acid and phenyl groups for the efficient conversion of levulinic acid or furfuryl alcohol to ethyl levulinate. ChemCatChem 2016, 8, 2037–2048. [Google Scholar] [CrossRef]
- Liu, X.; Maegawa, Y.; Goto, Y.; Hara, K.; Inagaki, S. Heterogeneous catalysis for water oxidation by an iridium complex immobilized on bipyridine-periodic mesoporous organosilica. Angew. Chem. Int. Ed. Engl. 2016, 55, 7943–7947. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.-Z.; Li, M.-M.; Gu, C.; Wan, M.; Duvail, J.-L.; Liu, Z.; Fan, Z. Recent advances in synthesis, physical properties and applications of conducting polymer nanotubes and nanofibers. Prog. Polym. Sci. 2011, 36, 1415–1442. [Google Scholar] [CrossRef]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; An, S.; Sun, Y.; Guo, Y. Efficient conversion of levulinic acid or furfuryl alcohol into alkyl levulinates catalyzed by heteropoly acid and ZrO2 bifunctionalized organosilica nanotubes. J. Catal. 2016, 333, 184–199. [Google Scholar] [CrossRef]
- Mandal, M.; Kruk, M. Family of single-micelle-templated organosilica hollow nanospheres and nanotubes synthesized through adjustment of organosilica/surgactant ratio. Chem. Mater. 2011, 24, 123–132. [Google Scholar] [CrossRef]
- Lim, M.H.; Stein, A. Comparative studies of grafting and direct syntheses of inorganic-organic hybrid mesoporous materials. Chem. Mater. 1999, 11, 3285–3295. [Google Scholar] [CrossRef]
- Wang, P.; Bai, S.; Zhao, J.; Su, P.; Yang, Q.; Li, C. Bifunctionalized hollow nanospheres for the one-pot synthesis of methyl isobutyl ketone from acetone. ChemSusChem 2012, 5, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; An, S.; Song, D.; Su, F.; Yang, X.; Guo, Y. Design of organosulfonic acid functionalized organosilica hollow nanospheres for efficient conversion of furfural alcohol to ethyl levulinate. Green Chem. 2015, 17, 1767–1778. [Google Scholar] [CrossRef]
- Russo, P.A.; Antunes, M.M.; Neves, P.; Wiper, P.V.; Fazio, E.; Neri, F.; Barreca, F.; Mafra, L.; Pillinger, M.; Pinna, N.; et al. Solid acids with SO3H groups and tunable surface properties: Versatile catalysts for biomass conversion. J. Mater. Chem. A 2014, 2, 11813–11824. [Google Scholar] [CrossRef]
- Hamoudi, S.; Kaliaguine, S. Sulfonic acid-functionalized periodic mesoporous organosilica. Microporous Mesoporous Mater. 2003, 59, 195–204. [Google Scholar] [CrossRef]
- Rác, B.; Hegyes, P.; Forgo, P.; Molnár, Á. Sulfonic acid-functionalized phenylene-bridged periodic mesoporous organosilicas as catalyst materials. Appl. Catal. A Gen. 2006, 299, 193–201. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, J.; Yang, J.; Yang, Q.; Li, C. Direct synthesis of highly ordered amine-functionalized mesoporous ethane-silica. Microporous Mesoporous Mater. 2008, 109, 172–183. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, F. Periodic mesoporous organosilica grafted palladium organometallic complex: Efficient heterogeneous catalyst for water-medium organic reactions. Appl. Organomet. Chem. 2010, 24, 767–773. [Google Scholar] [CrossRef]
- Wei, L.; Shi, D.; Zhou, Z.; Ye, P.; Wang, J.; Zhao, J.; Liu, L.; Chen, C.; Zhang, Y. Functionalized self-assembled monolayers on mesoporous silica nanoparticles with high surface coverage. Nanoscale Res. Lett. 2012, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Delidovich, I.; Palkovits, R. Impacts of acidity and textural properties of oxidized carbon materials on their catalytic activity for hydrolysis of cellobiose. Microporous Mesoporous Mater. 2016, 219, 317–321. [Google Scholar] [CrossRef]
- Fukuhara, K.; Nakajima, K.; Kitano, M.; Hayashi, S.; Hara, M. Synthesis and acid catalysis of zeolite-templated microporous carbons with SO3H groups. Phys. Chem. Chem. Phys. 2013, 15, 9343–9350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Xu, S.; Yang, Y.; Liu, J.; Wei, Y.; Yang, Q. Polystyrene sulphonic acid resins with enhanced acid strength via macromolecular self-assembly within confined nanospace. Nat. Commun. 2014, 5, 3170. [Google Scholar] [CrossRef] [PubMed]
- Karaki, M.; Karout, A.; Toufaily, J.; Rataboul, F.; Essayem, N.; Lebeau, B. Synthesis and characterization of acidic ordered mesoporous organosilica SBA-15: Application to the hydrolysis of cellobiose and insight into the stability of the acidic functions. J. Catal. 2013, 305, 204–216. [Google Scholar] [CrossRef]
- Yang, J.; Chen, W.; Ran, X.; Wang, W.; Fan, J.; Zhang, W.-X. Boric acid assisted formation of mesostructured silica: From hollow spheres to hierarchical assembly. RSC Adv. 2014, 4, 20069–20076. [Google Scholar] [CrossRef]









| Samples | BET Surface Area a (m2/g) | Pore Diameter b (nm) | Acid Content c (mmol/g) | Acid Density d (μmol/m2) | Oxidation Degree (%) e |
|---|---|---|---|---|---|
| SO3H0.1-Et-SNT | 647 | 5.5 | 0.48 | 0.74 | 75 |
| SO3H0.2-Et-SNT | 562 | 5.4 | 0.94 | 1.67 | 73 |
| SO3H0.3-Et-SNT | 380 | 5.6 | 1.66 | 4.37 | 66 |
| SO3H0.1-Ph-SNT | 537 | 4.3 | 0.42 | 0.78 | 81 |
| SO3H0.2-Ph-SNT | 485 | 4.8 | 0.89 | 1.84 | 63 |
| SO3H0.3-Ph-SNT | 421 | 4.8 | 1.41 | 3.35 | 77 |
| SO3H0.5-Ph-SNT | 231 | - | 2.02 | 8.74 | - |
| SO3H-SNT | 544 | 5.6 | 0.67 | 1.23 | - |
| Entry | Catalysts | Conversion of Cellobiose (%) b | Selectivity of Glucose (%) | TON c | TOF (h−1) d |
|---|---|---|---|---|---|
| 1 | SO3H0.1-Et-SNT | 65 | 93 | 8.4 | 7.5 |
| 2 | SO3H0.2-Et-SNT | 73 | 96 | 9.8 | 8.0 |
| 3 | SO3H0.3-Et-SNT | 87 | 89 | 12.2 | 10.4 |
| 4 | SO3H0.1-Ph-SNT | 70 | 90 | 8.8 | 8.1 |
| 5 | SO3H0.2-Ph-SNT | 79 | 92 | 10.1 | 11.1 |
| 6 | SO3H0.3-Ph-SNT | 92 | 96 | 12.3 | 12.1 |
| 7 | SO3H0.5-Ph-SNT | 78 | 91 | 9.8 | 7.0 |
| 8 | SO3H-SNT | 52 | 88 | 6.4 | 7.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, J.; Liu, X.; Zhu, X.; Wang, H.; Rostamnia, S.; Han, J. Well-Shaped Sulfonic Organosilica Nanotubes with High Activity for Hydrolysis of Cellobiose. Catalysts 2017, 7, 127. https://doi.org/10.3390/catal7050127
Sun J, Liu X, Zhu X, Wang H, Rostamnia S, Han J. Well-Shaped Sulfonic Organosilica Nanotubes with High Activity for Hydrolysis of Cellobiose. Catalysts. 2017; 7(5):127. https://doi.org/10.3390/catal7050127
Chicago/Turabian StyleSun, Jing, Xiao Liu, Xinli Zhu, Hua Wang, Sadegh Rostamnia, and Jinyu Han. 2017. "Well-Shaped Sulfonic Organosilica Nanotubes with High Activity for Hydrolysis of Cellobiose" Catalysts 7, no. 5: 127. https://doi.org/10.3390/catal7050127
APA StyleSun, J., Liu, X., Zhu, X., Wang, H., Rostamnia, S., & Han, J. (2017). Well-Shaped Sulfonic Organosilica Nanotubes with High Activity for Hydrolysis of Cellobiose. Catalysts, 7(5), 127. https://doi.org/10.3390/catal7050127