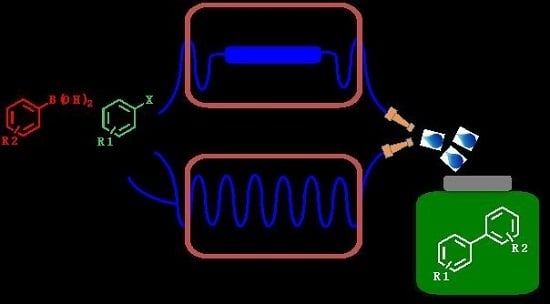
Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow
Abstract
:1. Introduction
2. Accepted Mechanism of Suzuki Cross-Coupling
3. Homogeneous Suzuki–Miyaura Cross-Coupling Reaction in Continuous Flow
4. Heterogeneous Suzuki–Miyaura Cross-Coupling Reaction in Continuous Flow
5. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Yin, L.; Liebscher, J. Carbon–carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Barnard, C. Palladium-catalyzed C–C coupling: Then and now. Platin. Met. Rev. 2008, 52, 38–45. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Sniekus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Agrofoglio, L.A.; Gillaizeau, I.; Saito, Y. Palladium-assisted routes to nucleosides. Chem. Rev. 2003, 103, 1875–1916. [Google Scholar] [CrossRef] [PubMed]
- Polshettiwar, V.; Len, C.; Fihri, A. Silica-supported palladium: Sustainable catalysts for cross-coupling reactions. Coord. Chem. Rev. 2009, 253, 2599–2626. [Google Scholar] [CrossRef]
- Heck, R.F. Acylation, methylation, and carboxyalkylation of olefins by group VIII metal derivatives. J. Am. Chem. Soc. 1968, 90, 5518–5526. [Google Scholar] [CrossRef]
- Mizoroki, T.; Mori, K.; Ozaki, A. Arylation of olefin with aryl iodide catalyzed by palladium. Bull. Chem. Soc. Jpn. 1971, 44, 581. [Google Scholar] [CrossRef]
- Heck, R.F.; Nolley, J.P. Palladium-catalyzed vinylic hydrogen substitution reactions with aryls benzyl, and styryl halides. J. Org. Chem. 1972, 37, 2320–2322. [Google Scholar] [CrossRef]
- Heck, R.F. Palladium-catalyzed reactions of organic halides with olefins. Acc. Chem. Res. 1979, 12, 146–151. [Google Scholar] [CrossRef]
- Dieck, H.A.; Heck, R.F. Organophosphinepalladium complexes as catalysts for vinylic hydrogen substitution reactions. J. Am. Chem. Soc. 1974, 96, 1133–1136. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Miyaura, N.; Yanagi, T.; Suzuki, A. The palladium-catalyzed cross-coupling reaction of phenylboronic acid with haloarenes in the presence of bases. Synt. Commun. 1981, 11, 513–519. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Suzuki, A. Recent advances in the cross-coupling reactions of organoboron derivatives with organic electrophiles, 1995–1998. J. Organomet. Chem. 1999, 576, 147–168. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions of organoboranes: An easy way to construct C–C bonds. Angew. Chem. Int. Ed. 2011, 50, 6722–6764. [Google Scholar] [CrossRef] [PubMed]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and brompyridines. Tetrahedron Lett. 1975, 16, 4467–4470. [Google Scholar] [CrossRef]
- Paterson, I.; Davies, R.D.; Marquez, R. Total synthesis of the Callipeltoside aglycon. Angew. Chem. Int. Ed. 2001, 40, 603–607. [Google Scholar] [CrossRef]
- Toyota, M.; Komori, C.; Ihara, M. A concise formal total synthesis of Mappicine and Nothapodytine B via an intramolecular hetero Diels-Alder reaction. J. Org. Chem. 2000, 65, 7110–7113. [Google Scholar] [CrossRef] [PubMed]
- Nicolaou, K.C.; Dai, W.M. Chemistry and biology of the enediyne anticancer antibiotics. Angew. Chem. Int. Ed. 1991, 30, 1387–1416. [Google Scholar] [CrossRef]
- Wu, R.; Schumm, J.S.; Pearson, D.L.; Tour, J.M. Convergent synthetic routes to orthogonally fused conjugated oligomers directed toward molecular scale electronic device applications. J. Org. Chem. 1996, 61, 6906–6921. [Google Scholar] [CrossRef] [PubMed]
- Milstein, D.; Stille, J.K. A general, selective, and facile method for ketone synthesis from acid chlorides and organotin compounds catalyzed by palladium. J. Am. Chem. Soc. 1978, 100, 3636–3638. [Google Scholar] [CrossRef]
- Milstein, D.; Stille, J.K. Palladium-catalyzed coupling of tetraorganotin compounds with aryl and benzyl halides. Synthetic utility and mechanism. J. Am. Chem. Soc. 1979, 101, 4992–4998. [Google Scholar] [CrossRef]
- Stille, J.K. The palladium-catalyzed cross-coupling reactions of organotin reagents with organic electrophiles. Angew. Chem. Int. Ed. 1986, 25, 508–524. [Google Scholar] [CrossRef]
- Espinet, P.; Echavarren, A.M. The mechanisms of Stille reaction. Angew. Chem. Ed. Int. 2004, 43, 4704–4734. [Google Scholar]
- Hiyama, T.; Hatanaka, Y. Palladium-catalyzed cross-coupling reaction of organometalloids through activation with fluoride ion. Pure Appl. Chem. 1994, 66, 1471–1478. [Google Scholar] [CrossRef]
- King, A.O.; Okukado, N.; Negishi, E. Highly general stereo-, regio-, and chemo-selective synthesis of terminal and internal conjugated enynes by the Pd-catalyzed reaction of alkynylzinc reagents with alkenyl halides. J. Chem. Soc. Chem. Commun. 1977, 683–684. [Google Scholar] [CrossRef]
- Tamao, K.; Sumitani, K.; Kumada, M. Selective carbon-carbon bond formation by cross-coupling of Grignard reagents with organic halides. Catalysis by nickel-phosphine complexes. J. Am. Chem. Soc. 1972, 94, 4374–4376. [Google Scholar] [CrossRef]
- Yamamura, M.; Moritani, I.; Murahashi, S.I. The reaction of σ-vinylpalladium complexes with alkyllithiums. Stereospecific syntheses of olefins from vinyl halides and alkyllithiums. J. Organomet. Chem. 1975, 91, C39–C42. [Google Scholar] [CrossRef]
- Hartwig, J.F. Handbook of Organopalladium Chemistry in Organic Synthesis; Negishi, E., Ed.; Wiley-Interscience: New York, NY, USA, 2003; p. 1051. [Google Scholar]
- Jiang, L.; Buchwald, S.L. Metal-Catalyzed Cross-Coupling Reactions; Meijere, A., Diederich, F., Eds.; Wiley-VCH: Weinheim, Germany, 2004; p. 699. [Google Scholar]
- Polshettiwar, V.; Decottignies, A.; Len, C.; Fihri, A. Suzuki-Miyaura cross-coupling reactions in aqueous media: Green and sustainable syntheses of biaryls. ChemSusChem 2010, 5, 502–522. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Luart, D.; Len, C.; Solhi, A.; Chevrin, C.; Polshettiwar, V. Suzuki-Miyaura cross-coupling reactions with low catalyst loading: A green and sustainable protocol in pure water. Dalton Trans. 2011, 40, 3116–3121. [Google Scholar] [CrossRef] [PubMed]
- Sartori, G.; Enderlin, G.; Herve, G.; Len, C. Highly effective synthesis of C-5-substituted 2′-deoxyuridine using Suzuki-Miyaura cross-coupling in water. Synthesis 2012, 44, 767–772. [Google Scholar] [CrossRef]
- Hassine, A.; Sebti, S.; Solhy, A.; Zahouily, M.; Len, C.; Hedhili, M.N.; Fihri, A. Palladium supported on natural phosphate: Catalyst for Suzuki coupling reactions in water. Appl. Catal. A Gen. 2013, 450, 13–18. [Google Scholar] [CrossRef]
- Sartori, G.; Enderlin, G.; Herve, G.; Len, C. New efficient approach for the ligand-free Suzuki-Miyaura reaction of 5-iodo-2′-deoxyuridine in water. Synthesis 2013, 45, 330–333. [Google Scholar] [CrossRef]
- Decottignies, A.; Fihri, A.; Azemar, G.; Djedaini-Pilard, F.; Len, C. Ligandless Suzuki-Miyaura reaction in neat water with or without native β-cyclodextrin as additive. Catal. Commun. 2013, 32, 101–107. [Google Scholar] [CrossRef]
- Gallagher-Duval, S.; Herve, G.; Sartori, G.; Enderlin, G.; Len, C. Improved microwave-assisted ligand free Suzuki-Miyaura cross-coupling of 5-iodo-2′-deoxyuridine in pure water. New J. Chem. 2013, 37, 1989–1995. [Google Scholar] [CrossRef]
- Enderlin, G.; Sartori, G.; Herve, G.; Len, C. Synthesis of 6-aryluridines via Suzuki-Miyaura cross-coupling reaction at room temperature under aerobic ligand-free conditions in neat water. Tetrahedron Lett. 2013, 54, 3374–3377. [Google Scholar] [CrossRef]
- Herve, G.; Sartori, G.; Enderlin, G.; Mackenzie, G.; Len, C. Palladium-catalyzed Suzuki reaction in aqueous solvents applied to unprotected nucleosides and nucleotides. RSC Adv. 2014, 4, 18558–18594. [Google Scholar] [CrossRef]
- Herve, G.; Len, C. First ligand-free, microwave-assisted, Heck cross-coupling reaction in pure water on a nucleoside—Application to the synthesis of antiviral BVDU. RSC Adv. 2014, 4, 46926–46929. [Google Scholar] [CrossRef]
- Lussier, T.; Herve, G.; Enderlin, G.; Len, C. Original access to 5-aryluracils from 5-iodo-2′-deoxyuridine via a microwave-assisted Suzuki-Miyaura cross-coupling/deglycosylation sequence in pure water. RSC Adv. 2014, 4, 46218–46223. [Google Scholar] [CrossRef]
- Hassine, A.; Bouhrara, M.; Sebti, S.; Solhy, A.; Luart, D.; Len, C.; Fihri, A. Natural phosphate-supported palladium: A highly efficient and recyclable catalyst for the Suzuki-Miyaura coupling under microwave irradiation. Curr. Org. Chem. 2014, 18, 3141–3148. [Google Scholar] [CrossRef]
- Haswell, S.J.; Watts, P. Green chemistry: Synthesis in micro reactors. Green Chem. 2003, 5, 240–249. [Google Scholar] [CrossRef]
- Frost, C.G.; Mutton, L. Heterogeneous catalytic syntheis using microreactor technology. Green Chem. 2010, 12, 1687–1703. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous flow reactors: A perspective. Green Chem. 2012, 14, 38–54. [Google Scholar] [CrossRef]
- Newman, S.G.; Jensen, K.F. The role of flow in green chemistry and engineering. Green Chem. 2013, 15, 1456–1472. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P. Continuous process technology: A tool for sustainable production. Green Chem. 2014, 16, 55–62. [Google Scholar] [CrossRef]
- Vaccaro, L.; Lanari, D.; Marrochi, A.; Strappaveccia, G. Flow approaches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Falb, S.; Tomaiuolo, G.; Perazzo, A.; Hodgson, P.; Yaseneva, P.; Zakrzewski, J.; Guido, S.; Lapkin, A.; Woodward, R.; Meadows, R.E. A continuous process for Buchwald-Hartwig amination at micro-, lab-, and mesoscale using a novel reactor concept. Org. Process Res. Dev. 2016, 20, 558–565. [Google Scholar]
- Gemoets, H.P.L.; Hessel, V.; Noel, T. Aerobic C–H olefination of indoles via a cross-dehydrogenative coupling in continuous flow. Org. Lett. 2014, 16, 5800–5803. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.R.; Plucinski, P.; Frost, C.G. Robust and reusable supported palladium catalysts for cross-coupling reactions in flow. Catal. Sci. Technol. 2014, 4, 948–954. [Google Scholar] [CrossRef]
- Bourne, S.L.; O’Brien, M.; Kasinathan, S.; Koos, P.; Tolstoy, P.; Hu, D.X.; Bates, R.W.; Martin, B.; Schenkel, B.; Ley, S.V. Flow chemistry syntheses of styrenes, unsymmetrical stilbenes and branched aldehydes. ChemCatChem 2013, 5, 159–172. [Google Scholar] [CrossRef]
- Peeva, L.; da Silva Burgal, J.; Vartak, S.; Livingston, A.G. Experimental strategies for increasing the catalyst turnover number in a continuous Heck coupling reaction. J. Catal. 2013, 306, 190–201. [Google Scholar] [CrossRef]
- Sharma, S.; Basavaraju, K.C.; Singh, A.K.; Kim, D.P. Continuous recycling of homogeneous Pd/cu catalysts for cross-coupling reactions. Org. Lett. 2014, 16, 3974–3977. [Google Scholar] [CrossRef] [PubMed]
- Peeva, L.; Arbour, J.; Livingston, A. On the potential of organic solvent nanofiltration in continuous Heck coupling reactions. Org. Process. Res. Dev. 2013, 17, 967–975. [Google Scholar] [CrossRef]
- Domier, R.C.; Moore, J.N.; Shaughnessy, K.H.; Hartman, R.L. Kinetic analysis of aqueous-phase Pd-catalyzed, Cu-free direct arylation of terminal alkynes using a hydrophilic ligand. Org. Process. Res. Dev. 2013, 17, 1262–1271. [Google Scholar] [CrossRef]
- Tukacs, J.M.; Jones, R.V.; Darvas, F.; Dibo, G.; Lezsak, G.; Mika, L.T. Synthesis of γ-valerolactone using a continuous flow reactor. RSC Adv. 2013, 3, 16283–16287. [Google Scholar] [CrossRef]
- Yang, G.R.; Bae, G.; Choe, J.; Lee, S.; Song, K.H. Silica-supported palladium-catalyzed Hiyama cross-coupling reactions using continuous flow system. Bull. Korean Chem. Soc. 2010, 31, 250–252. [Google Scholar] [CrossRef]
- Phan, N.T.S.; Brown, D.H.; Styring, P. A facile method for catalyst immobilization on silica: Nickel-catalyzed Kumada reactions in mini-continuous flow and batch reactors. Green Chem. 2004, 6, 526–532. [Google Scholar] [CrossRef]
- Alonso, N.; Zane Miller, L.; Munoz, J.D.M.; Alcazar, J.; Tyler McQuade, D. Continuous synthesis of organozinc halides coupled to Negishi reactions. Adv. Synth. Catal. 2014, 356, 3737–3741. [Google Scholar] [CrossRef]
- Tan, L.M.; Sem, Z.Y.; Chong, W.Y.; Liu, X.; Hendra; Kwan, W.L.; Ken Lee, C.L. Continuous flow Sonogashira C–C coupling using a heterogeneous palladium-copper dual reactor. Org. Lett. 2013, 15, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Xing, C.H.; Bouobda Tsemo, G.; Hu, Q.S. t-Bu3P-coordinated 2-phenylaniline-based palladacycle complex as a precatalyst for the Suzuki cross-coupling polymerization of aryl dibromides with aryldiboronic acids. ACS Macro Lett. 2013, 2, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Schulte, N.; Breuning, E.; Spreitzer, H. Method for the Production of Polymers. U.S. Patent 20,080,207,851 A1, 28 December 2004. [Google Scholar]
- Seyler, H.; Jones, D.J.; Holmes, A.B.; Wong, W.W.H. Continuous flow synthesis of conjugated polymers. Chem. Commun. 2012, 48, 1598–1600. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Subbiah, J.; Geraghty, P.B.; Chen, M.; Purushothaman, B.; Chen, X.; Qin, T.; Vak, D.; Scholes, F.H.; Watkins, S.E.; et al. Development of a high-performance donor-acceptor conjugated polymer: Synergy in materials and device optimization. Chem. Mater. 2016, 28, 3481–3487. [Google Scholar] [CrossRef]
- Mitchell, V.D.; Wong, W.W.H. Synthetic Methods for Conjugated Polymer and Carbon Materials; Leclerc, M., Morin, J.-F., Eds.; Wiley-VCH: Weinheim, Germany, 2017; p. 65. [Google Scholar]
- Amatore, C.; Jutand, A.; Le Duc, G. Kinetic data for the transmetallation/reductive elimination in palladium-catalyzed Suzuki-Miyaura reactions: Unexpected triple role of hydroxide ions used as base. Chem. Eur. J. 2011, 17, 2492–2503. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Jutand, A.; Le Duc, G. Mechanistic origin of antagonist effects of usual anionic bases (OH−, CO32−) as modulated by their countercations (Na+, Cs+, K+) in palladium-catalyzed Suzuki-Miyaura reactions. Chem. Eur. J. 2012, 18, 6616–6625. [Google Scholar] [CrossRef] [PubMed]
- Carrow, B.P.; Hartwig, J.F. Distinguishing between pathways for transmetalation in Suzuki-Miyara reactions. J. Am. Chem. Soc. 2011, 133, 2116–2119. [Google Scholar] [CrossRef] [PubMed]
- Cantillo, D.; Kappe, C.O. Immobilized Transition Metals as Catalysts for Cross-Couplings in Continuous Flow—A Critical Assessment of the Reaction Mechanism and Metal Leaching. ChemCatChem 2014, 6, 3286–3305. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of catalysis on the stability of metallic nanoparticles: Suzuki reaction catalyzed by PVP-palladium nanoparticles. J. Am. Chem. Soc. 2003, 125, 8340–8347. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, R.; El-Sayed, M.A. Effect of catalytic activity on the metallic nanoparticle size distribution: Electron-transfer reaction between Fe(CN)6 and thiosulfate ions catalyzed by PVP-platinium nanoparticles. J. Phys. Chem. B 2003, 107, 12416–12424. [Google Scholar] [CrossRef]
- De Vries, A.H.M.; Mulders, J.; Mommers, J.H.M.; Henderickx, H.J.W.; De Vries, J.G. Homeopathic ligand-free palladium as a catalyst in the Heck reaction. A comparison with a palladacycle. Org. Lett. 2003, 5, 3285–3288. [Google Scholar] [CrossRef] [PubMed]
- De Vries, J.G. A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton Trans. 2006, 21, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Bhanage, B.M.; Shirai, M.; Arai, M. Heck reactions of iodobenzene and methyl acrylate with conventional supported palladium catalysts in the presence of organic and/and inorganic bases without ligands. Chem. Eur. J. 2000, 6, 843–848. [Google Scholar] [CrossRef]
- Bhanage, B.M.; Shirai, M.; Arai, M. Heterogeneous catalyst system for Heck reaction using supported ethylene glycol phase Pd/TPPTS catalyst with inorganic base. J. Mol. Catal. A 1999, 145, 69–74. [Google Scholar] [CrossRef]
- Reetz, M.T.; Westermann, E. Phosphane-free palladium-catalyzed coupling reactions: The decisive role of Pd nanoparticles. Angew. Chem. Int. Ed. 2000, 39, 165–168. [Google Scholar] [CrossRef]
- Reetz, M.T.; Helbig, W.; Quasier, S.A.; Stimming, U.; Breuer, N.; Vogel, R. Visualization of surfactants on nanostructured palladium clusters by a combination of STM and high-resolution TEM. Science 1995, 267, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Thathagar, M.B.; Ten Elshof, J.E.; Rothenberg, G. Pd nanoclusters in C–C coupling reactions: Proof of leaching. Angew. Chem. Int. Ed. 2006, 45, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, A.V.; Holuigue, A.; Thathagar, M.B.; Ten Elhof, J.E.; Rothenberg, G. Ion- and atom-leaching mechanisms from palladium nanoparticles in cross-coupling reactions. Chem. Eur. J. 2007, 13, 6908–6913. [Google Scholar] [CrossRef] [PubMed]
- Ananikov, V.P.; Beletskaya, I.P. Toward the ideal catalyst: From atomic centers to a “cocktail” of catalysts. Organometallics 2012, 31, 1595–1604. [Google Scholar] [CrossRef]
- Kashin, A.S.; Ananikov, V.P. Catalytic C–C and C–heteroatom bond formation reactions: In Situ generated of preformed catalysts? Complicated mechanistic picture behing well-known experimental procedures. J. Org. Chem. 2013, 78, 11117–11125. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Pellegatti, L.; Oberli, M.A.; Buchwald, S.L. Continuous-flow synthesis of biaryls enabled by multistep solid-handling in a lithiation/borylation/Suzuki-Miyaura cross-coupling sequence. Angew. Chem. Int. Ed. 2011, 50, 10665–10669. [Google Scholar] [CrossRef] [PubMed]
- Kabri, Y.; Gellis, A.; Vanelle, P. Synthesis of original 2-substituted 4-arylquinazolines by microwave-irradiated Suzuki-Miyaura cross-coupling reactions. Eur. J. Org. Chem. 2009, 2009, 4059–4066. [Google Scholar] [CrossRef]
- Gill, G.S.; Grobelny, D.W.; Chaplin, J.H.; Flynn, B.L. An efficient synthesis and substitution of 3-aroyl-2-bromobenzo[b]furans. J. Org. Chem. 2008, 73, 1131–1134. [Google Scholar] [CrossRef] [PubMed]
- Organ, M.; Calimsiz, S.; Sayah, M.; Hoi, K.; Lough, A. Pd-PEPPSI-IPent: An active, sterically demanding cross-coupling catalyst and its application in the synthesis of tetra-ortho-substituted biaryls. Angew. Chem. Int. Ed. 2009, 48, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.; Chen, Y. One-pot oxidation and bromination of 3,4-diaryl-2,5-dihydrothiophenes using Br2: Synthesis and application of 3,4-diaryl-2,5-dibromothiophenes. J. Org. Chem. 2007, 72, 6901–6904. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Haketa, Y.; Nakanishi, T. Aryl-substituted C3-bridged oligopyrroles as anion receptors for formation of supramolecular organogels. J. Am. Chem. Soc. 2007, 129, 13661–13674. [Google Scholar] [CrossRef] [PubMed]
- James, C.A.; Coelho, A.L.; Gevaert, M.; Forgione, P.; Snieckus, V. Combined directed ortho and remote metalation-Suzuki cross-cooupling strategies. Efficient synthesis of heteroaryl-fused benzopyrannones from biaryl O-carbamates. J. Org. Chem. 2009, 74, 4094–4103. [Google Scholar] [CrossRef] [PubMed]
- Giodiano, C.; Coppi, L.; Minisci, F. Process for the Preparation of 5-(2,4-difluorophenyl)-salicylic Acid. U.S. Patent 5,312,975, 17 May 1994. [Google Scholar]
- Hannah, J.; Ruyle, W.V.; Jones, H.; Matzuk, A.R.; Kelly, K.W.; Witzel, B.E.; Holtz, W.J.; Houser, R.A.; Shen, T.Y.; Sarett, L.H. Novel analgesic-antiinflammatory salicylates. J. Med. Chem. 1978, 21, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Reizman, B.J.; Wang, Y.M.; Buchwald, S.L.; Jensen, K.F. Suzuki-Miyaura cross-coupling optimization enabled by automated feedback. React. Chem. Eng. 2016, 1, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Teci, M.; Tilley, M.; McGuire, M.A.; Organ, M.G. Using anilines as masked cross-coupling partners: Design of a telescoped three-step flow diazotization, iododediazotization, cross-coupling process. Chem. Eur. J. 2016, 22, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ormerod, D.; Lefevre, N.; Dorbec, M.; Eyskens, I.; Vloemans, P.; Duyssens, K.; Diez de la Torre, V.; Kaval, N.; Merkul, E.; Sergeyev, S.; et al. Potential of homogeneous Pd catalyst separation by ceramic membranes. Application to downstream and continuous flow processes. Org. Process Res. Dev. 2016, 20, 911–920. [Google Scholar] [CrossRef]
- Cole, K.P.; Campbell, B.M.; Forst, M.B.; McClary Groh, J.; Hess, M.; Johnson, M.D.; Miller, R.D.; Mitchell, D.; Polster, C.S.; Reizman, B.J.; et al. An automated intermittent flow approach to continuous Suzuki coupling. Org. Process Res. Dev. 2016, 20, 820–830. [Google Scholar] [CrossRef]
- Hattori, T.; Tsubone, A.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Palladium on carbon-catalyzed Suzuki-Miyaura coupling reaction using an efficient and continuous flow system. Catalysts 2015, 5, 18–25. [Google Scholar] [CrossRef]
- Brinkley, K.W.; Burkholder, M.; Siamaki, A.R.; Belecki, K.; Gupton, B.F. The continuous synthesis and application of graphene supported palladium nanoparticles: A highly effective catalyst for Suzuki-Miyaura cross-coupling reactions. Green Process Synth. 2015, 4, 241–246. [Google Scholar] [CrossRef]
- Bolton, K.F.; Canty, A.J.; Deverell, J.A.; Guijt, R.M.; Hilder, E.F.; Rodemann, T.; Smith, J.A. Macroporous monolith supports for continuous flow capillary microreactors. Tetrahedron Lett. 2006, 47, 9321–9324. [Google Scholar] [CrossRef]
- Nagaki, A.; Hirose, K.; Moriwaki, Y.; Mitamura, K.; Matsukawa, K.; Ishizuka, N.; Yoshida, J. Integration of borylation of aryllithiums and Suzuki-Miyaura coupling using monolithic Pd catalyst. Catal. Sci. Technol. 2016, 6, 4690–4694. [Google Scholar] [CrossRef]
- Shil, A.K.; Guha, N.R.; Sharma, D.; Das, P. A solid supported palladium(0) nano/microparticle catalyzed ultrasound induced continuous flow technique for large scale Suzuki reactions. RSC Adv. 2013, 3, 13671–13676. [Google Scholar] [CrossRef]
- Pascanu, V.; Hansen, P.R.; Bermejo-Gomez, A.; Ayats, C.; Platero-Prats, A.E.; Johansson, M.J.; Pericas, M.A.; Martin-Matute, B. Highly functionalized biaryls via Suzuki-Miyaura cross coupling catalyzed by Pd@MOF under batch and continuous flow regimes. ChemSusChem 2015, 8, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Huskens, J.; Verboom, W. Dendrimer-encapsulated Pd nanoparticles as catalysts for C–C cross-couplings in flow microreactors. Org. Biomol. Chem. 2015, 13, 4953–4959. [Google Scholar] [CrossRef] [PubMed]
- Ricciardi, R.; Huskens, J.; Holtkamp, M.; Karst, U.; Verboom, W. Dendrimer-encapsulated palladium nanoparticles for continuous-flow Suzuki-Miyaura cross-coupling reactions. ChemCatChem 2015, 7, 936–942. [Google Scholar] [CrossRef]
- Rehm, T.H.; Bogdan, A.; Hofmann, C.; Lob, P.; Shifrina, Z.; Morgan, D.G.; Bronstein, L.M. Proof of concept: Magnetic fixation of dendron-functionalized iron oxide nanoparticles containing Pd nanoparticles for continuous-flow Suzuki coupling reactions. ACS Appl. Mater. Interfaces 2015, 7, 27254–27261. [Google Scholar] [CrossRef] [PubMed]
- Seto, H.; Yoneda, T.; Morii, T.; Hoshino, Y.; Miura, Y. Membrane reactor immobilized with palladium-moaded polymer nanogel for continuous-flow Suzuki coupling reaction. AIChE J. 2015, 61, 582–589. [Google Scholar] [CrossRef]
- Gu, Y.; Favier, I.; Pradel, C.; Gin, D.L.; Lahitte, J.F.; Noble, R.D.; Gomez, M.; Remigny, J.C. High catalytic efficiency of palladium nanoparticles immobilized in a polymer membrane containing poly(ionic liquid) in Suzuki-Miyaura cross-coupling reaction. J. Membr. Sci. 2015, 492, 331–339. [Google Scholar] [CrossRef]
- Dai, Y.; Formo, E.; Li, H.; Xue, J.; Xia, Y. Surface-functionalized electrospun titania nanofibers for the scavenging and recycling of precious metal ions. ChemSusChem 2016, 9, 2912–2916. [Google Scholar] [CrossRef] [PubMed]
- Munoz, J.D.M.; Alcazar, J.; de la Hoz, A.; Diaz-Ortiz, A. Cross-coupling in flow using supported catalysts: Mild, clean, efficient and sustainable Suzuki-Miyaura coupling in a single pass. Adv. Synth. Catal. 2012, 354, 3456–3460. [Google Scholar] [CrossRef]
- Noel, T.; Kuhn, S.; Musacchio, A.J.; Jensen, K.F.; Buchwald, S.L. Suzuki-Miyaura cross-coupling reactions in flow: Multistep synthesis enabled by a microfluidic extraction. Angew. Chem. Int. Ed. 2011, 50, 5943–5946. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Goessler, W.; Cantillo, D.; Kappe, C.O. Benchmarking immobilized Di- and Triaryphosphine palladium catalysts for continuous-flow cross-coupling reactions: Efficiency, durability, and metal leaching studies. ACS Catal. 2015, 5, 1303–1312. [Google Scholar] [CrossRef]
- Pandarus, V.; Gingras, G.; Beland, F.; Ciriminna, R.; Pagliaro, M. Process intensification of the Suzuki-Miyaura reaction over sol-gel entrapped catalyst SiliaCat DPP-Pd under conditions of continuous flow. Org. Process Res. Dev. 2014, 18, 1550–1555. [Google Scholar] [CrossRef]
- Pandarus, V.; Gingras, G.; Beland, F.; Ciriminnia, R.; Pagliaro, M. Fast and clean borylation of aryl halides under flow using sol-gel entrapped SiliaCat DPP-Pd. Org. Process Res. Dev. 2014, 18, 1556–1559. [Google Scholar] [CrossRef]
- Pandarus, V.; Ciriminnia, R.; Gingras, G.; Beland, F.; Drobod, M.; Jina, O.; Pagliaro, M. Greening heterogeneous catalysis for fine chemicals. Tetrahedron Lett. 2013, 54, 1129–1132. [Google Scholar] [CrossRef]
- Goncalves, R.S.B.; de Oliveira, A.B.V.; Sindra, H.C.; Archanjo, B.S.; Mendoza, M.E.; Carneiro, L.S.A.; Buarque, C.D.; Esteves, P.M. Heterogeneous catalysis by covalent organic frameworks (COF): Pd(OAc)2@COF-300 in cross-coupling reactions. ChemCatChem 2016, 8, 743–750. [Google Scholar] [CrossRef]
- Trinh, T.N.; Hizartzidis, L.; Lin, A.J.S.; Harman, D.G.; McCluskey, A.; Gordon, C.P. An efficient continuous flow approach to furnish furan-based biaryls. Org. Biomol. Chem. 2014, 12, 9562–9571. [Google Scholar] [CrossRef] [PubMed]
































© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Len, C.; Bruniaux, S.; Delbecq, F.; Parmar, V.S. Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow. Catalysts 2017, 7, 146. https://doi.org/10.3390/catal7050146
Len C, Bruniaux S, Delbecq F, Parmar VS. Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow. Catalysts. 2017; 7(5):146. https://doi.org/10.3390/catal7050146
Chicago/Turabian StyleLen, Christophe, Sophie Bruniaux, Frederic Delbecq, and Virinder S. Parmar. 2017. "Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow" Catalysts 7, no. 5: 146. https://doi.org/10.3390/catal7050146
APA StyleLen, C., Bruniaux, S., Delbecq, F., & Parmar, V. S. (2017). Palladium-Catalyzed Suzuki–Miyaura Cross-Coupling in Continuous Flow. Catalysts, 7(5), 146. https://doi.org/10.3390/catal7050146