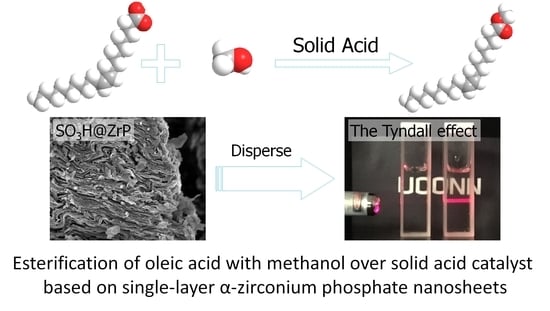
Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Experimental
4.1. Materials
4.2. Preparation of ZrP Nanosheets
4.3. Preparation and Characterization of Solid Acid
4.4. Esterification of Oleic Acid with Methanol
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Lee, A.F.; Bennett, J.A.; Manayil, J.C.; Wilson, K. Heterogeneous catalysis for sustainable biodiesel production via esterification and transesterification. Chem. Soc. Rev. 2014, 43, 7887–7916. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Iglesias, J.; Morales, G. Heterogeneous acid catalysts for biodiesel production: Current status and future challenges. Green Chem. 2009, 11, 1285–1308. [Google Scholar] [CrossRef]
- Noshadi, I.; Kanjilal, B.; Du, S.; Bollas, G.M.; Suib, S.L.; Provatas, A.; Liu, F.; Parnas, R.S. Catalyzed production of biodiesel and bio-chemicals from brown grease using Ionic Liquid functionalized ordered mesoporous polymer. Appl. Energy 2014, 129, 112–122. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Chung, K.-H.; Park, B.-G. Esterification of oleic acid in soybean oil on zeolite catalysts with different acidity. J. Ind. Eng. Chem. 2009, 15, 388–392. [Google Scholar] [CrossRef]
- Shin, H.Y.; An, S.H.; Sheikh, R.; Park, Y.H.; Bae, S.-H. Transesterification of used vegetable oils with a Cs-doped heteropolyacid catalyst in supercritical methanol. Fuel 2012, 96, 572–578. [Google Scholar] [CrossRef]
- Pan, Y.; Alam, M.A.; Wang, Z.; Wu, J.; Zhang, Y.; Yuan, Z. Enhanced esterification of oleic acid and methanol by deep eutectic solvent assisted Amberlyst heterogeneous catalyst. Bioresour. Technol. 2016, 220, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ilgen, O. Investigation of reaction parameters, kinetics and mechanism of oleic acid esterification with methanol by using Amberlyst 46 as a catalyst. Fuel Process. Technol. 2014, 124, 134–139. [Google Scholar] [CrossRef]
- Reis, S.C.M.D.; Lachter, E.R.; Nascimento, R.S.V.; Rodrigues, J.A.; Reid, M.G. Transesterification of Brazilian vegetable oils with methanol over ion-exchange resins. J. Am. Oil Chem. Soc. 2005, 82, 661–665. [Google Scholar] [CrossRef]
- Liu, T.; Li, Z.; Li, W.; Shi, C.; Wang, Y. Preparation and characterization of biomass carbon-based solid acid catalyst for the esterification of oleic acid with methanol. Bioresour. Technol. 2013, 133, 618–621. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Salley, S.O.; Ng, K.S. Simultaneous transesterification and esterification of unrefined or waste oils over ZnO-La2O3 catalysts. Appl. Catal. Gen. 2009, 353, 203–212. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Sun, D.; Clearfield, A.; Sue, H.-J. Preparation of exfoliated epoxy/α-zirconium phosphate nanocomposites containing high aspect ratio nanoplatelets. Chem. Mater. 2007, 19, 1749–1754. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Sue, H.-J.; Clearfield, A. Preparation of α-zirconium phosphate nanoplatelets with wide variations in aspect ratios. New J. Chem. 2007, 31, 39–43. [Google Scholar] [CrossRef]
- Pica, M.; Donnadio, A.; Capitani, D.; Vivani, R.; Troni, E.; Casciola, M. Advances in the chemistry of nanosized zirconium phosphates: A new mild and quick route to the synthesis of nanocrystals. Inorg. Chem. 2011, 50, 11623–11630. [Google Scholar] [CrossRef] [PubMed]
- Pica, M.; Donnadio, A.; Mariangeloni, G.; Zuccaccia, C.; Casciola, M. A combined strategy for the synthesis of double functionalized α-zirconium phosphate organic derivatives. New J. Chem. 2016, 40, 8390–8396. [Google Scholar] [CrossRef]
- Pica, M.; Nocchetti, M.; Ridolfi, B.; Donnadio, A.; Costantino, F.; Gentili, P.L.; Casciola, M. Nanosized zirconium phosphate/AgCl composite materials: A new synergy for efficient photocatalytic degradation of organic dye pollutants. J. Mater. Chem. A 2015, 3, 5525–5534. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, R.; Ding, F.; Brittain, A.D.; Liu, J.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Sulfonic acid-functionalized α-zirconium phosphate single-layer nanosheets as a strong solid acid for heterogeneous catalysis applications. ACS Appl. Mater. Interfaces 2014, 6, 7417–7425. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, A.; Wang, Z.; Chen, M.; Wang, W.; Sun, L.; Liu, X. Titanium functionalized α-zirconium phosphate single layer nanosheets for photocatalyst applications. RSC Adv. 2015, 5, 93969–93978. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Huang, R.; Zhang, M.; Xiao, M.; Meng, Y.; Sun, L. Covalently immobilized ionic liquids on single layer nanosheets for heterogeneous catalysis applications. Dalton Trans. 2017, 46, 13126–13134. [Google Scholar] [CrossRef] [PubMed]
- Pica, M.; Donnadio, A.; Casciola, M. Starch/zirconium phosphate composite films: Hydration, thermal stability, and mechanical properties. Starch-Stärke 2012, 64, 237–245. [Google Scholar] [CrossRef]
- Casciola, M.; Alberti, G.; Donnadio, A.; Pica, M.; Marmottini, F.; Bottino, A.; Piaggio, P. Gels of zirconium phosphate in organic solvents and their use for the preparation of polymeric nanocomposites. J. Mater. Chem. 2005, 15, 4262–4267. [Google Scholar] [CrossRef]
- Boo, W.J.; Sun, L.; Liu, J.; Clearfield, A.; Sue, H.-J. Effective intercalation and exfoliation of nanoplatelets in epoxy via creation of porous pathways. J. Phys. Chem. C 2007, 111, 10377–10381. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.J.; Browning, R.L.; Sue, H.-J.; Clearfield, A. Effect of Crystallinity on the Intercalation of Monoamine in α-Zirconium Phosphate Layer Structure. Chem. Mater. 2005, 17, 5606–5609. [Google Scholar] [CrossRef]
- Pica, M.; Donnadio, A.; Troni, E.; Capitani, D.; Casciola, M. Looking for New Hybrid Polymer Fillers: Synthesis of Nanosized α-Type Zr(IV) Organophosphonates through an Unconventional Topotactic Anion Exchange Reaction. Inorg. Chem. 2013, 52, 7680–7687. [Google Scholar] [CrossRef] [PubMed]
- Boo, W.J.; Sun, L.; Liu, J.; Clearfield, A.; Sue, H.-J.; Mullins, M.J.; Pham, H. Morphology and mechanical behavior of exfoliated epoxy/α-zirconium phosphate nanocomposites. Comp. Sci. Tech. 2007, 67, 262–269. [Google Scholar] [CrossRef]
- Sun, L.; Boo, W.-J.; Clearfield, A.; Sue, H.-J.; Pham, H.Q. Barrier properties of model epoxy nanocomposites. J. Membr. Sci. 2008, 318, 129–136. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; Kirumakki, S.R.; Schwerdtfeger, E.D.; Howell, R.J.; Al-Bahily, K.; Miller, S.A.; Clearfield, A.; Sue, H.-J. Polypropylene nanocomposites based on designed synthetic nanoplatelets. Chem. Mater. 2009, 21, 1154–1161. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Xiao, M.; Meng, Y.; Sun, L. Designing Supported Ionic Liquids (ILs) within Inorganic Nanosheets for CO2 Capture Applications. ACS Appl. Mater. Interfaces 2016, 8, 5547–5555. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Lin, K.-Y.; Kung, C.-C.; Jhuo, J.-W.; Zhou, Y.; Liu, J.; Sun, L. Intercalated polyfluorinated Pd complexes in α-zirconium phosphate for Sonogashira and Heck reactions. RSC Adv. 2014, 4, 27329–27336. [Google Scholar] [CrossRef]
- Hu, H.; Martin, J.C.; Xiao, M.; Southworth, C.S.; Meng, Y.; Sun, L. Immobilization of ionic liquids in layered compounds via mechanochemical intercalation. J. Phys. Chem. C 2011, 115, 5509–5514. [Google Scholar] [CrossRef]
- Kaschak, D.M.; Johnson, S.A.; Hooks, D.E.; Kim, H.-N.; Ward, M.D.; Mallouk, T.E. Chemistry on the edge: A microscopic analysis of the intercalation, exfoliation, edge functionalization, and monolayer surface tiling reactions of α-zirconium phosphate. J. Am. Chem. Soc. 1998, 120, 10887–10894. [Google Scholar] [CrossRef]
- Alberti, G. Syntheses, crystalline structure, and ion-exchange properties of insoluble acid salts of tetravalent metals and their salt forms. Acc. Chem. Res. 1978, 11, 163–170. [Google Scholar] [CrossRef]
- Alberti, G.; Casciola, M.; Costantino, U. Inorganic ion-exchange pellicles obtained by delamination of α-zirconium phosphate crystals. J. Colloid Interface Sci. 1985, 107, 256–263. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Noshadi, I.; Ding, H.; Liu, J.; Parnas, R.S.; Clearfield, A.; Xiao, M.; Meng, Y.; Sun, L. Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification. Catalysts 2018, 8, 17. https://doi.org/10.3390/catal8010017
Zhou Y, Noshadi I, Ding H, Liu J, Parnas RS, Clearfield A, Xiao M, Meng Y, Sun L. Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification. Catalysts. 2018; 8(1):17. https://doi.org/10.3390/catal8010017
Chicago/Turabian StyleZhou, Yingjie, Iman Noshadi, Hao Ding, Jingjing Liu, Richard S. Parnas, Abraham Clearfield, Min Xiao, Yuezhong Meng, and Luyi Sun. 2018. "Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification" Catalysts 8, no. 1: 17. https://doi.org/10.3390/catal8010017
APA StyleZhou, Y., Noshadi, I., Ding, H., Liu, J., Parnas, R. S., Clearfield, A., Xiao, M., Meng, Y., & Sun, L. (2018). Solid Acid Catalyst Based on Single-Layer α-Zirconium Phosphate Nanosheets for Biodiesel Production via Esterification. Catalysts, 8(1), 17. https://doi.org/10.3390/catal8010017