Laccase-Catalyzed Reduction of Oxygen at Electrodes Modified by Carbon Nanotubes with Adsorbed Promazine or Acetosyringone
Abstract
:1. Introduction
2. Results and Discussion
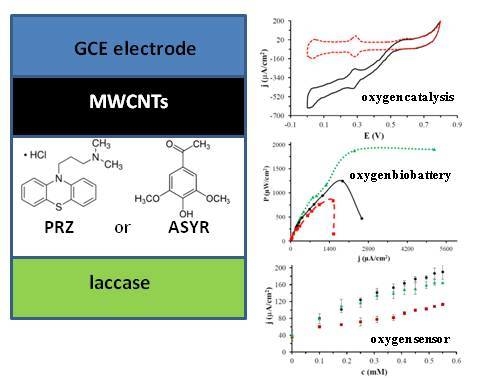
2.1. Electrochemical Studies
2.2. Biobattery Studies
2.3. Oxygen Sensor
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Electrochemical Instrumentation and Procedures
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Barton, S.C.; Gallaway, J.; Atanassov, P. Enzymatic biofuel cells for implantable and microscale devices. Chem. Rev. 2004, 104, 4867–4886. [Google Scholar] [CrossRef]
- Cinquin, P.; Gondran, C.; Giroud, F.; Mazabrard, S.; Pellissier, A.; Boucher, F.; Alcaraz, J.P.; Gorgy, K.; Lenouvel, F.; Mathe, S.; et al. A glucose biofuel cell implanted in rats. PLoS ONE 2010, 5, e10476. [Google Scholar] [CrossRef]
- Falk, M.; Alcalde, M.; Bartlett, P.N.; De Lacey, A.L.; Gorton, L.; Gutierrez-Sanchez, C.; Haddad, R.; Kilburn, J.; Leech, D.; Ludwig, R.; et al. Self-powered wireless carbohydrate/oxygen sensitive biodevice based on radio signal transmission. PLoS ONE 2014, 10, e10910. [Google Scholar] [CrossRef]
- Majdecka, D.; Draminska, S.; Stolarczyk, K.; Kizling, M.; Krysinski, P.; Golimowski, J.; Biernat, J.F.; Bilewicz, R. Sandwich biobattery with enzymatic cathode and zinc anode integrated with sensor. J. Electrochem. Soc. 2015, 162, F555–F559. [Google Scholar] [CrossRef]
- Skunik-Nuckowska, M.; Grzejszczyk, K.; Stolarczyk, K.; Bilewicz, R.; Kulesza, P.J. Integration of supercapacitors with enzymatic biobatteries toward more effective pulse-powered use in small-scale energy harvesting devices. J. Appl. Electrochem. 2014, 44, 497–507. [Google Scholar] [CrossRef] [Green Version]
- Zebda, A.; Cosnier, S.; Alcaraz, J.P.; Holzinger, M.; Le Goff, A.; Gondran, C.; Boucher, F.; Giroud, F.; Gorfy, K.; Lamraoi, H.; et al. Single glucose biofuel cells implanted in rats power electronic devices. Sci. Rep. 2013, 3, 1516. [Google Scholar] [CrossRef] [PubMed]
- Conzuelo, F.; Ruff, A.; Schuhmann, W. Self-powered bioelectrochemical devices. Curr. Opin. Electrochem. 2018. [Google Scholar] [CrossRef]
- Katz, E.; Buckmann, A.F.; Willner, I. Self-powered enzyme-based biosensors. J. Am. Chem. Soc. 2001, 123, 10752–10753. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, J. Biofuel cells for self-powered electrochemical biosensing and logic biosensing: A review. Electroanalysis 2012, 24, 197–209. [Google Scholar] [CrossRef]
- Himmel, M.E.; Baker, J.O.; Overend, R.P.; Palmore, G.T.R.; Whitesides, G.M. Microbial and enzymatic biofuel cells, Enzymatic conversion of biomass for fuels production. ACS Books Am. Chem. Soc. 1994, 14, 271–290. [Google Scholar]
- Leech, D.; Kavanagh, P.; Schuhmann, W. Enzymatic fuel cell: Recent progress. Electrochim. Acta 2012, 84, 223–234. [Google Scholar] [CrossRef]
- Cracknell, J.A.; Vincent, K.A.; Armstrong, F.A. Enzymes as working or inspirational electrocatalysts for fuel cells and electrolysis. Chem. Rev. 2008, 108, 2439–2461. [Google Scholar] [CrossRef] [PubMed]
- Gellett, W.; Kesmez, M.; Schumacher, J.; Akers, N.; Minteer, S.D. Biofuel cells for portable power. Electroanalysis 2010, 22, 727–731. [Google Scholar] [CrossRef]
- Heller, A. Miniature biofuel cells. Phys. Chem. Chem. Phys. 2004, 6, 209–216. [Google Scholar] [CrossRef]
- Willner, I.; Yan, Y.M.; Willner, B.; Tel-Vered, R. Integrated enzyme-based biofuel cells—A review. Fuel Cells 2009, 9, 7–24. [Google Scholar] [CrossRef]
- Ansari, S.A.; Husain, Q. Potential applications of enzymes immobilized on/in nano materials: A review. Biotechnol. Adv. 2012, 30, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Antiochia, R.; Gorton, L. A new osmium-polymer modified screen-printed graphene electrode for fructose detection. Sens. Actuators B 2014, 195, 287–293. [Google Scholar] [CrossRef]
- Gupta, M.N.; Kaloti, M.; Kapoor, M.; Solanki, K. Nanomaterials as matrices for enzyme immobilization. Artif. Cells Blood Substit. Immobil. Biotechnol. 2011, 39, 98–109. [Google Scholar] [CrossRef]
- Kizling, M.; Stolarczyk, K.; Tammela, P.; Wang, Z.; Nyholm, L.; Golimowski, J.; Bilewicz, R. Bioelectrodes based on pseudocapacitive cellulose/polypyrrole composite improve performance of biofuel cell. Bioelectrochemistry 2016, 112, 184–190. [Google Scholar] [CrossRef]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Strømme, M. Toward flexible polymer and paper-based energy storage devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar] [CrossRef]
- So, K.; Kawai, S.; Hamano, Y.; Kitazumi, Y.; Shirai, O.; Hibi, M.; Ogawa, J.; Kano, K. Improvement of a direct electron transfer-type fructose/dioxygen biofuel cell with substrate-modified biocathode. Phys. Chem. Chem. Phys. 2014, 16, 4823–4829. [Google Scholar] [CrossRef] [PubMed]
- Żelechowska, K.; Stolarczyk, K.; Łyp, D.; Rogalski, J.; Roberts, K.P.; Bilewicz, R.; Biernat, J.F. Aryl and N- arylamide carbon nanotubes for electrical coupling of laccase to electrodes in biofuel cells and biobatteries. Biocybern. Biomed. Eng. 2013, 33, 235–245. [Google Scholar] [CrossRef]
- Feng, W.; Ji, P. Enzymes immobilized on carbon nanotubes. Biotechnol. Adv. 2011, 29, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Barrow, C.J.; Puri, M. Nanobiotechnology as a novel paradigm for enzyme immobilisation and stabilisation with potential applications in biodiesel production. Appl. Microbiol. Biotechnol. 2013, 97, 23–39. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, P.; Leech, D. Mediated electron transfer in glucose oxidising enzyme electrodes for application to biofuel cells: Recent progress and perspectives. Phys. Chem. Chem. Phys. 2013, 15, 4859–4869. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, K.; Kizling, M.; Majdecka, D.; Żelechowska, K.; Biernat, J.F.; Rogalski, J.; Bilewicz, R. Biobatteries and biofuel cells with biphenylated carbon nanotubes. J. Power Sources 2014, 249, 263–269. [Google Scholar] [CrossRef]
- Parimi, N.S.; Umasankar, Y.; Atanassov, P.; Ramasamy, R.P. Kinetic and mechanistic parameters for laccase catalyzed direct electrochemical oxygen reduction reaction. ACS Catal. 2012, 2, 38–44. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Nazaruk, E.; Rogalski, J.; Bilewicz, R. Mediatorless catalytic oxygen reduction at boron-doped diamond electrodes. Electrochem. Commun. 2007, 9, 115–118. [Google Scholar] [CrossRef]
- Palmore, G.T.R.; Kim, H.H. Electro-enzymatic reduction of dioxygen to water in the cathode compartment of a biofuel cell. J. Electroanal. Chem. 1999, 464, 110–117. [Google Scholar] [CrossRef]
- Mao, F.; Mano, N.; Heller, A. Long tethers binding redox centers to polymer backbones enhance electron transport in enzyme “wiring” hydrogels. J. Am. Chem. Soc. 2003, 125, 4951–4957. [Google Scholar] [CrossRef]
- Heller, A. Electron-conducting redox hydrogels: Design, characteristics and synthesis. Curr. Opin. Chem. Biol. 2006, 10, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Foye, W.O. Principios de Quımica Farmacéutica; Editorial Reverte: Barcelona, Spanish, 1984; p. 222. [Google Scholar]
- Lara, F.J.; Garcya-Campana, A.M.; Ales-Barrero, F.; Bosque-Sendra, J.M. Determination of thiazinamium, promazine and promethazine in pharmaceutical formulations using a CZE method. Anal. Chim. Acta 2005, 535, 101–108. [Google Scholar] [CrossRef]
- Baker, C.J.; Mock, N.M.; Whitaker, B.D.; Roberts, D.P.; Rice, C.P.; Deahl, K.L.; Aver’Yanov, A.A. Involvement of acetosyringone in plant-pathogen recognition. Biochem. Biophys. Res. Commun. 2005, 328, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, C.; Tzanov, T.; Gubitz, G.M.; Cavaco-Paulo, A. Voltammetric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry 2002, 58, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, K.; Sepunaru, L.; Compton, R.G. Innovative catalyst design for the oxygen reduction reaction for fuel cells. Chem. Sci. 2016, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kruusenberg, I.; Alexeyeva, N.; Tammeveski, K. The pH-dependence of oxygen reduction on multi-walled carbon nanotube modified glassy carbon electrodes. Carbon 2009, 47, 651–658. [Google Scholar] [CrossRef]
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A green catalyst for the biosynthesis of poly-phenols. Crit. Rev. Biotechnol. 2018, 38, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Feng, C. Electrochemical polymerization of hydroquinone on graphite felt as a pseudocapacitive material for application in a microbial fuel cell. Polymers 2017, 9, 220. [Google Scholar] [CrossRef]
- Romero-Guido, C.; Baez, A.; Torres, E. Dioxygen activation by laccases: Green chemistry for fine chemical synthesis. Catalysts 2018, 8, 223. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Łyp, D.; Żelechowska, K.; Biernat, J.F.; Rogalski, J.; Bilewicz, R. Arylated carbon nanotubes for biobatteries and biofuel cells. Electrochim. Acta 2012, 79, 74–81. [Google Scholar] [CrossRef]
- Rola, B.; Karaskiewicz, M.; Majdecka, D.; Mazur, I.; Bilewicz, R.; Rogalski, J.; Ohga, S. Scale up of Cerrena unicolor laccase production. J. Fac. Agric. Kyushu Univ. 2013, 58, 231–238. [Google Scholar]






| Electrode GCE | jbcg (µA/cm2) | jcat (µA/cm2) | jcat − jbcg (µA/cm2) |
|---|---|---|---|
| MWCNTs/Lc | −16.0 ± 0.8 | −346.0 ± 8.0 | −330.0 ± 8.2 |
| MWCNTs/PRZ/Lc | −57.7 ± 7.1 | −480.8 ± 30.4 | −423.0 ± 27.0 |
| MWCNTs/ASYR/Lc | −128.3 ± 8.3 | −629.6 ± 8.0 | −501.4 ± 16.2 |
| (A) | |||||
| Electrode GCE | P (µW/cm2) | J (µA/cm2) | E (V) | R (kΩ) | OCP (V) |
| MWCNTs/Lc | 822.1 ± 17.0 | 1521.7 ± 15,8 | 0.540 ± 0.006 | 5 | 1.570 ± 0.012 |
| MWCNTs/PRZ/Lc | 1307.2 ± 90.0 | 1918.3 ± 66.2 | 0.681 ± 0.024 | 5 | 1.544 ± 0.035 |
| MWCNTs/ASYR/Lc | 1853.9 ± 125.3 | 2284.3 ± 77.5 | 0.811 ± 0.028 | 5 | 1.637 ± 0.032 |
| (B) | |||||
| Electrode GCE | P (µW/cm2) | J (µA/cm2) | E (V) | R (kΩ) | OCP (V) |
| MWCNTs/Lc | 2.9 ± 0.8 | 89.7± 3.7 | 0.032 ± 0.005 | 5 | 0.230 ± 0.061 |
| MWCNTs/PRZ/Lc | 6.0 ± 1.2 | 129.5 ± 12.7 | 0.046 ± 0.005 | 5 | 0.215 ± 0.025 |
| MWCNTs/ASYR/Lc | 22.1 ± 4.0 | 248.9 ± 22.6 | 0.088 ± 0.008 | 5 | 0.186 ± 0.011 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olszewski, B.; Stolarczyk, K. Laccase-Catalyzed Reduction of Oxygen at Electrodes Modified by Carbon Nanotubes with Adsorbed Promazine or Acetosyringone. Catalysts 2018, 8, 414. https://doi.org/10.3390/catal8100414
Olszewski B, Stolarczyk K. Laccase-Catalyzed Reduction of Oxygen at Electrodes Modified by Carbon Nanotubes with Adsorbed Promazine or Acetosyringone. Catalysts. 2018; 8(10):414. https://doi.org/10.3390/catal8100414
Chicago/Turabian StyleOlszewski, Bartłomiej, and Krzysztof Stolarczyk. 2018. "Laccase-Catalyzed Reduction of Oxygen at Electrodes Modified by Carbon Nanotubes with Adsorbed Promazine or Acetosyringone" Catalysts 8, no. 10: 414. https://doi.org/10.3390/catal8100414
APA StyleOlszewski, B., & Stolarczyk, K. (2018). Laccase-Catalyzed Reduction of Oxygen at Electrodes Modified by Carbon Nanotubes with Adsorbed Promazine or Acetosyringone. Catalysts, 8(10), 414. https://doi.org/10.3390/catal8100414