Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst
Abstract
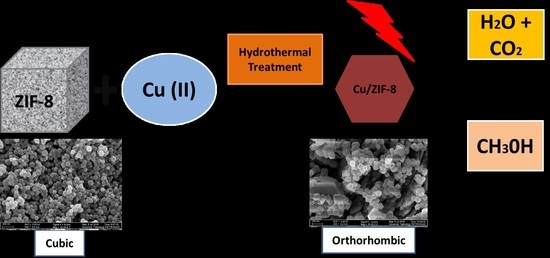
:1. Introduction
2. Results and Discussion
2.1. Effect of Solvent Concentration on Cu/ZIF-8
2.2. Effect of Copper Loading on ZIF-8
2.3. Photocatalytic Activity
3. Materials and Methods
3.1. Preparation of ZIF-8
3.2. Synthesis of Catalysts
3.3. Characterization
3.4. Photocatalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

| Sample | Crystal Size | Crystal Structure | Band Gap Energy (eV) | VB Potential (V) | CB Potential (V) | Methanol Yield (µmol/L·g) |
|---|---|---|---|---|---|---|
| ZIF-8 | 32 nm | Cubic | 4.89 | 2.41 | −2.48 | No yield |
| 2Cu/ZIF-8N4 | 200 nm | Orthorhombic | 4.58 | 2.14 | −2.44 | 0.655 |
| 2Cu/ZIF-8N3 | 114 nm | Tetragonal | 3.50 | 1.6 | −1.9 | 16.19 |
| 2Cu/ZIF-8N2 | 50 nm | Orthorhombic | 1.75 | 0.72 | −1.02 | 35.82 |
| 1Cu/ZIF-8N2 | 38 nm | Orthorhombic | 1.80 | 0.75 | −1.05 | 2.6 |
| 3Cu/ZIF-8N2 | 82 nm | Orthorhombic | 1.65 | 0.67 | −0.97 | 27.38 |
References
- Ma, S.; Sadakiyo, M.; Heima, M.; Luo, R.; Haasch, R.T.; Gold, J.I.; Yamauchi, M.; Kenis, P.J. Electroreduction of carbon dioxide to hydrocarbons using bimetallic Cu-Pd catalysts with different mixing patterns. J. Am. Chem. Soc. 2016, 139, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Fujishima, A.; Konishi, S.; Honda, K. Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 1979, 277, 637–638. [Google Scholar] [CrossRef]
- Kimfung, L.; Martin, D.; Junwang, T. Conversion of solar energy to fuels by inorganic heterogeneous systems. Chin. J. Catal. 2011, 32, 879–890. [Google Scholar]
- Li, K.; An, X.; Park, K.H.; Khraisheh, M.; Tang, J. A critical review of CO2 photoconversion: Catalysts and reactors. Catal. Today 2014, 224, 3–12. [Google Scholar] [CrossRef]
- Xie, S.; Wang, Y.; Zhang, Q.; Fan, W.; Deng, W.; Wang, Y. Photocatalytic reduction of CO2 with H2O: Significant enhancement of the activity of Pt–TiO2 in CH4 formation by addition of MgO. Chem. Commun. 2013, 49, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xie, S.; Zhang, Q.; Tian, Z.; Wang, Y. Carbon dioxide-enhanced photosynthesis of methane and hydrogen from carbon dioxide and water over Pt-promoted polyaniline–TiO2 nanocomposites. Chem. Commun. 2015, 51, 13654–13657. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Kumar, P.; Sharma, O.P.; Jain, S.L.; Khatri, O.P. Reduced graphene oxide–CuO nanocomposites for photocatalytic conversion of CO2 into methanol under visible light irradiation. Appl. Catal. B 2016, 181, 352–362. [Google Scholar] [CrossRef]
- Shi, H.; Chen, G.; Zhang, C.; Zou, Z. Polymeric g-C3N4 coupled with NaNbO3 nanowires toward enhanced photocatalytic reduction of CO2 into renewable fuel. ACS Catal. 2014, 4, 3637–3643. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Han, L.; Li, C.; Zhang, S. Photocatalytic reduction of CO2 with H2O on CuO/TiO2 catalysts. Energy Sources 2016, 38, 420–426. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, K.; Zhang, Z.; Li, M.; Li, B. Effect of contact interface between TiO2 and g-C3N4 on the photoreactivity of g-C3N4/TiO2 photocatalyst:(0 0 1) vs. (1 0 1) facets of TiO2. Appl. Catal. B. 2015, 164, 420–427. [Google Scholar]
- Eddaoudi, M.; Sava, D.F.; Eubank, J.F.; Adil, K.; Guillerm, V. Zeolite-like metal–organic frameworks (ZMOFs): Design, synthesis, and properties. Chem. Soc. Rev. 2015, 44, 228–249. [Google Scholar] [CrossRef] [PubMed]
- McGuire, C.V.; Forgan, R.S. The surface chemistry of metal–organic frameworks. Chem. Commun. 2015, 51, 5199–5217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-C.; Li, J.-R.; Lv, X.-L.; Zhang, Y.-Q.; Guo, G. Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ. Sci. 2014, 7, 2831–2867. [Google Scholar] [CrossRef]
- Meyer, K.; Ranocchiari, M.; van Bokhoven, J.A. Metal organic frameworks for photo-catalytic water splitting. Energy Environ. Sci. 2015, 8, 1923–1937. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X. Multifunctional metal–organic frameworks for photocatalysis. Small 2015, 11, 3097–3112. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, J.; Song, J.; Li, J.; Liu, J.; Wu, T.; Zhang, P.; Han, B. Ru nanoparticles immobilized on metal–organic framework nanorods by supercritical CO2-methanol solution: Highly efficient catalyst. Green Chem. 2011, 13, 2078–2082. [Google Scholar] [CrossRef]
- Zhang, T.; Lin, W. Metal–organic frameworks for artificial photosynthesis and photocatalysis. Chem. Soc. Rev. 2014, 43, 5982–5993. [Google Scholar] [CrossRef] [PubMed]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Low, Z.-X.; Li, L.; Razmjou, A.; Wang, K.; Yao, J.; Wang, H. ZIF-8/Zn2GeO4 nanorods with an enhanced CO2 adsorption property in an aqueous medium for photocatalytic synthesis of liquid fuel. J. Mater. Chem. A 2013, 1, 11563–11569. [Google Scholar] [CrossRef]
- Jing, H.-P.; Wang, C.-C.; Zhang, Y.-W.; Wang, P.; Li, R. Photocatalytic degradation of methylene blue in ZIF-8. RSC Adv. 2014, 4, 54454–54462. [Google Scholar] [CrossRef]
- Tran, U.P.; Le, K.K.; Phan, N.T. Expanding applications of metal–organic frameworks: Zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the knoevenagel reaction. ACS Catal. 2011, 1, 120–127. [Google Scholar] [CrossRef]
- Schejn, A.; Aboulaich, A.; Balan, L.; Falk, V.; Lalevée, J.; Medjahdi, G.; Aranda, L.; Mozet, K.; Schneider, R. Cu2+-doped zeolitic imidazolate frameworks (ZIF-8): Efficient and stable catalysts for cycloadditions and condensation reactions. Catal. Sci. Technol. 2015, 5, 1829–1839. [Google Scholar] [CrossRef]
- Yang, X.; Wen, Z.; Wu, Z.; Luo, X. Synthesis of ZnO/ZIF-8 hybrid photocatalysts derived from ZIF-8 with enhanced photocatalytic activity. Inorg. Chem. Front. 2018, 5, 687–693. [Google Scholar] [CrossRef]
- Dutta, T.; Bagchi, D.; Pal, S.K. Bimetallic zeolitic imidazolate framework as an active excipient of curcumin under physiological condition. Biomed. Phys. Eng. Exp. 2018, 4, 055004. [Google Scholar] [CrossRef]
- Wang, S.; Yao, W.; Lin, J.; Ding, Z.; Wang, X. Cobalt Imidazolate Metal–Organic Frameworks Photosplit CO2 under Mild Reaction Conditions. Angew. Chem. 2014, 126, 1052–1056. [Google Scholar] [CrossRef]
- Wang, Z.; Teramura, K.; Hosokawa, S.; Tanaka, T. Photocatalytic conversion of CO2 in water over Ag-modified La2Ti2O7. Appl. Catal. B 2015, 163, 241–247. [Google Scholar] [CrossRef]
- Slamet, H.W.N.; Purnama, E.; Riyani, K.; Gunlazuardi, J. Effect of copper species in a photocatalytic synthesis of methanol from carbon dioxide over copper-doped titania catalysts. World Appl. Sci. J. 2009, 6, 112–122. [Google Scholar]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on Photocatalytic CO2 Reduction over NH2-Uio-66 (Zr) and Its Derivatives: Towards a Better Understanding of Photocatalysis on Metal–Organic Frameworks. Chem. Eur. J. 2013, 19, 14279–14285. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Xin, F.; Chen, J.; Wang, Y.; Yin, X.; Shao, X. Selective photocatalytic reduction of CO2 to methanol in CuO-loaded NaTaO3 nanocubes in isopropanol. Beilstein J. Nanotech. 2016, 7, 776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xian, S.; Xi, H.; Wang, H.; Li, Z. Improvement of CO2 adsorption on ZIF-8 crystals modified by enhancing basicity of surface. Chem. Eng. Sci. 2011, 66, 4878–4888. [Google Scholar] [CrossRef]
- Park, K.S.; Ni, Z.; Côté, A.P.; Choi, J.Y.; Huang, R.; Uribe-Romo, F.J.; Chae, H.K.; O’Keeffe, M.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cravillon, J.; Nayuk, R.; Springer, S.; Feldhoff, A.; Huber, K.; Wiebcke, M. Controlling zeolitic imidazolate framework nano-and microcrystal formation: Insight into crystal growth by time-resolved in situ static light scattering. Chem. Mater. 2011, 23, 2130–2141. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, Y.; Zeng, G.; Zhao, L.; Lai, Z. Rapid synthesis of zeolitic imidazolate framework-8 (ZIF-8) nanocrystals in an aqueous system. Chem. Commun. 2011, 47, 2071–2073. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Othman, M.H.D.; Jilani, A.; Ismail, A.F.; Hashim, H.; Jaafar, J.; Rahman, M.A.; Rehman, G.U. Economical, environmental friendly synthesis, characterization for the production of zeolitic imidazolate framework-8 (ZIF-8) nanoparticles with enhanced CO2 adsorption. Arab. J. Chem. 2018, 11, 1072–1083. [Google Scholar] [CrossRef]
- Wu, H.; Qian, X.; Zhu, H.; Ma, S.; Zhu, G.; Long, Y. Controlled synthesis of highly stable zeolitic imidazolate framework-67 dodecahedra and their use towards the templated formation of a hollow Co3O4 catalyst for CO oxidation. RSC Adv. 2016, 6, 6915–6920. [Google Scholar] [CrossRef]
- Chary, K.V.R.; Sagar, G.V.; Naresh, D.; Seela, K.K.; Sridhar, B. Characterization and Reactivity of Copper Oxide Catalysts Supported on TiO2−ZrO2. J. Phy. Chem. B 2005, 109, 9437–9444. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Shaharun, M.S.; Kait, C.F. Characterization of copper (ii)-zirconium (iv)-imidazolate framework synthesized by hydrothermal method. IIOAB J. 2016, 7, 5. [Google Scholar]
- Jeyalakshmi, V.; Mahalakshmy, R.; Krishnamurthy, K.; Viswanathan, B. Titania Based Catalysts for Photoreduction of Carbon Dioxide: Role of Modifiers; NISCAIR-CSIR: New Delhi, India, 2012. [Google Scholar]
- Li, H.; Lei, Y.; Huang, Y.; Fang, Y.; Xu, Y.; Zhu, L.; Li, X. Photocatalytic reduction of carbon dioxide to methanol by Cu2O/SiC nanocrystallite under visible light irradiation. J. Nat. Gas Chem. 2011, 20, 145–150. [Google Scholar] [CrossRef]
- Uddin, M.R.; Khan, M.R.; Rahman, M.W.; Yousuf, A.; Cheng, C.K. Photocatalytic reduction of CO2 into methanol over CuFe2O4/TiO2 under visible light irradiation. React. Kinet. Mech. Catal. 2015, 116, 589–604. [Google Scholar] [CrossRef]
- Grosu, Y.; Renaudin, G.; Eroshenko, V.; Nedelec, J.-M.; Grolier, J.-P. Synergetic effect of temperature and pressure on energetic and structural characteristics of {ZIF-8+ water} molecular spring. Nanoscale 2015, 7, 8803–8810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Q.; Liu, C.; Shan, X.; Chen, X.; Dai, W.; Fu, X. The promoted effect of a metal-organic frameworks (ZIF-8) on Au/TiO2 for CO oxidation at room temperature both in dark and under visible light irradiation. Appl. Catal. B 2018, 224, 283–294. [Google Scholar] [CrossRef]
- Qin, S.; Xin, F.; Liu, Y.; Yin, X.; Ma, W. Photocatalytic reduction of CO2 in methanol to methyl formate over CuO–TiO2 composite catalysts. J. Colloid Interface Sci. 2011, 356, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lin, Q.; Zhang, M.; He, G.; Sun, Z. Microstructure, optical properties, and catalytic performance of Cu2 O-modified ZnO nanorods prepared by electrodeposition. Nanoscale Res. Lett. 2015, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Dimitrijevic, N.M.; Chen, L.; Rajh, T.; Gray, K.A. Role of surface/interfacial Cu2+ sites in the photocatalytic activity of coupled CuO−TiO2 nanocomposites. J. Phy. Chem. C 2008, 112, 19040–19044. [Google Scholar] [CrossRef]
- Colon, G.; Maicu, M.; Hidalgo, M.S.; Navio, J. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Li, Y.; Cao, L.; Guo, Y. Asymmetric interface band alignments of Cu2O/ZnO and ZnO/Cu2O heterojunctions. J. Alloy. Compd. 2013, 578, 143–147. [Google Scholar] [CrossRef]
- Li, J.; Luo, D.; Yang, C.; He, S.; Chen, S.; Lin, J.; Zhu, L.; Li, X. Copper (II) imidazolate frameworks as highly efficient photocatalysts for reduction of CO2 into methanol under visible light irradiation. J. Solid State Chem. 2013, 203, 154–159. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Sun, Q.; Wang, Z.; Huang, B.; Dai, Y.; Qin, X.; Zhang, X. Chemical adsorption enhanced CO2 capture and photoreduction over a copper porphyrin based metal organic framework. ACS Appl. Mater. Interfaces 2013, 5, 7654–7658. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.; Mumtaz, A.; Mumtaz, M.; Mutalib, M.A.; Shaharun, M.S.; Abdullah, B. Visible light driven CO2 reduction to methanol by Cu-porphyrin impregnated mesoporous Ti-MCM-48. J. Mol. Liq. 2018. [Google Scholar] [CrossRef]
- Wang, J.; Chen, K.; Shen, Y.; Wang, X.; Guo, Y.; Zhou, X.; Bai, R. Enhanced photocatalytic degradation for organic pollutants by a novel m-Bi2O4/Bi2O2CO3 photocatalyst under visible light. Res. Chem. Intermediat. 2018, 44, 3061–3079. [Google Scholar] [CrossRef]
- Ren, M.; Valsaraj, K. Inverse opal titania on optical fiber for the photoreduction of CO2 to CH3OH. Int. J. Chem. React. Eng. 2009, 7. [Google Scholar] [CrossRef]
- Usubharatana, P.; McMartin, D.; Veawab, A.; Tontiwachwuthikul, P. Photocatalytic process for CO2 emission reduction from industrial flue gas streams. Ind. Eng. Chem. Res. 2006, 45, 2558–2568. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 surfaces: Principles, mechanisms, and selected results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Tahir, M.; Tahir, B.; Amin, N.A.S.; Alias, H. Selective photocatalytic reduction of CO2 by H2O/H2 to CH4 and CH3OH over Cu-promoted In2O3/TiO2 nanocatalyst. Appl. Surf. Sci. 2016, 389, 46–55. [Google Scholar] [CrossRef]











| Sr. No. | Catalyst | Reactant | Preparation Method | Reactor Condition | Product/Yield | Ref. |
|---|---|---|---|---|---|---|
| 1 | Cu porphyrin-based MOF | CO2 and Solution of the triethylamine | Hydrothermal | 300 W Xe lamp | 5.97 µmol/g·h CH3OH | [50] 2013 |
| 2 | ZIF-8/Zn2GeO4 | CO2 and the Solution of sodium sulfite | Impregnation | 500 W Xe arc lamp | 2.44 µmol/g CH3OH | [19] 2013 |
| 3 | CuIm | CO2 and solutions of sodium hydroxide and sodium sulfite | Hydrothermal | 500 W, Xe lamp | 1712.7 µmol/g CH3OH | [48] 2013 |
| 4 | Ti-MCM-48(25)CuTPP | CO2 and solutions of sodium hydroxide and sodium sulfite | Impregnation | 500 W Xenon Lamp | 297.06 μmol/g CH3OH | [51] 2018 |
| 5 | TiO2P25 | CO2 and solution of potassium hydrogen carbonate | Commercial | 10 W UV Lamp | 800 µmol/g·catal CH3OH | [27] 2009 |
| 6 | 2Cu/ZIF-8N2 | CO2 and solutions of sodium hydroxide and sodium sulfite | Hydrothermal | 500 W Xenon Lamp | 35.82 µmol/L·g CH3OH | This study |
| S. No. | Copper Metal Loading (mmol) | ZIF-8 Loading (mmol) | Ammonium Hydroxide Concentration (M) | Catalyst Notation |
|---|---|---|---|---|
| 1. | 2 | 4 | 4 | 2Cu/ZIF-8N4 |
| 2. | 2 | 4 | 3 | 2Cu/ZIF-8N3 |
| 3. | 2 | 4 | 2 | 2Cu/ZIF-8N2 |
| 4. | 1 | 4 | 2 | 1Cu/ZIF-8N2 |
| 5. | 3 | 4 | 2 | 3Cu/ZIF-8N2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyal, S.; Shaharun, M.S.; Kait, C.F.; Abdullah, B.; Ameen, M. Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst. Catalysts 2018, 8, 581. https://doi.org/10.3390/catal8120581
Goyal S, Shaharun MS, Kait CF, Abdullah B, Ameen M. Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst. Catalysts. 2018; 8(12):581. https://doi.org/10.3390/catal8120581
Chicago/Turabian StyleGoyal, Sonam, Maizatul Shima Shaharun, Chong Fai Kait, Bawadi Abdullah, and Mariam Ameen. 2018. "Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst" Catalysts 8, no. 12: 581. https://doi.org/10.3390/catal8120581
APA StyleGoyal, S., Shaharun, M. S., Kait, C. F., Abdullah, B., & Ameen, M. (2018). Photoreduction of Carbon Dioxide to Methanol over Copper Based Zeolitic Imidazolate Framework-8: A New Generation Photocatalyst. Catalysts, 8(12), 581. https://doi.org/10.3390/catal8120581