Compositing Two-Dimensional Materials with TiO2 for Photocatalysis
Abstract
:1. Introduction
2. 2D-Material Modified TiO2
2.1. Graphene Modified TiO2
2.1.1. The Synthesis of Graphene/TiO2 Composites
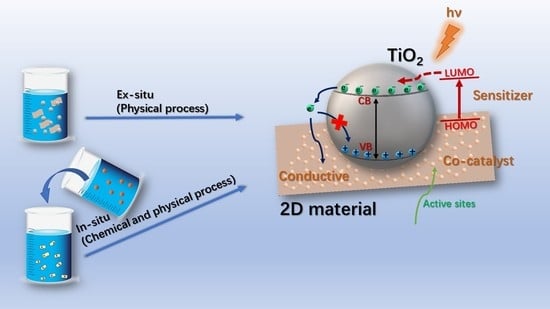
- Ex-situ hybridization. The common procedure for ex-situ hybridization is to mix GO and modified TiO2 with physical process such as ultrasound sonication and heat treatments. Rahmatollah et al. [62] reported a facile one-step solvothermal method to synthesize the TiO2-graphene composite sheets by dissolving different mass ratios of GO and TiO2 nanoparticles in anhydrous ethanol solution. Ultrasound irradiation was used to disperse the GO. Finally, a six-fold enhancement was observed in the photocurrent response compared to the improved photoelectrochemical performance (3%) with the pure TiO2. Florina et al. [63] prepared graphene/TiO2-Ag based composites as electrode materials. Similarly, GO suspensions were mixed with prepared TiO2-Ag nanoparticles in NaOH solution. The suspensions were sonicated, dried and subjected to thermal treatment. However, the control of modification between the TiO2 and graphene may lead to a decreased interaction between these two parts [64].
- In-situ growth. The in-situ growth method is widely used to prepare graphene-based composite materials, and the method can effectively avoid clustering of nanoparticles on the surface of graphene. According to different preparation process, it might be divided into reduction method, electrochemical deposition method, hydrothermal method and sol-gel method.
- Reduction method. Usually, in a reduction method, GO and TiO2 metal salts are mixed as precursors. By controlling the hydrolysis of the precursor, TiO2 crystal nucleus grows on GO, while GO is reduced to obtain graphene-based TiO2 composite materials [65]. In addition to the chemical reduction method, other commonly used reduction methods are photocatalytic reduction [66] and microwave-assisted chemical reduction [67].
- Electrochemical deposition method. In an electrochemical deposition method, graphene or reduced graphene is used as a working electrode in a dielectric solution containing a metal precursor or its compound [68].
- Sol-gel method. The sol-gel method takes titanium alkoxide or titanium chloride as precursors, and it can be uniformly bonded with oxygen group on graphene, polycondensed to form a gel. Then TiO2 nanoparticles are formed through calcining [71,72]. The sol-gel method can obtain loaded nanoparticles with higher uniformity of dispersion.
2.1.2. The Role of Graphene in TiO2 Photocatalysis
2.2. Graphdiyne Modified TiO2
2.2.1. The Synthesis of GD/TiO2 Composites
2.2.2. The Role of GD in TiO2 Photocatalysis
2.3. C3N4 Modified TiO2
2.3.1. The Synthesis of g-C3N4/TiO2 Composites
- In the ex-situ way, both g-C3N4 and TiO2 materials are pre-prepared, which can be integrated through physical process such as ball milling [94], solvent evaporation [95,96], etc. Though physical process is easy to operate under moderate conditions, some flaws also exist such as ununiformly dispersing and unstable structure.
- The in-situ method uses one of the materials as a substrate and then the other material grows on the surface of the substrate. For g-C3N4/TiO2 composites, both materials can be regarded as substrates.
- When used as substrates, g-C3N4 is pre-prepared by calcinations of precursors. Solvothermal/hydrothermal method is most common for the next step. After mixing g-C3N4 and titanates in a certain solvent, the solution is well dispersed and sealed in the Teflon-lined autoclave, followed by a solvothermal/hydrothermal treatment [97,98,99]. Furthermore, Atomic Layer Deposition (ALD) was applied to form thin TiO2 films on g-C3N4 substrates. ALD involves the surface of a substrate exposed alternately to alternating precursor flow. Then the precursor molecule reacts with the surface in a self-limiting way, which guarantees that the reaction stops as all the reactive sites on the substrate reacted with the precursors. It is an effective way to control the thickness and homogeneity of deposited layer [100].
- When TiO2 was used as substrates, calcination is widely used for the convenience and easy operation. In this process, the solid mixture of TiO2 and pure urea or melamine or dicyandiamide powder are calcinated under fixed temperature to obtain g-C3N4/TiO2 composites. Before calcination, the two components should be evenly dispersed by sonication [101], stirring [102], or grounding [103]. Recently, Tan et al. [104] reported another facile one-step way to prepare nanostructured g-C3N4/TiO2 composite. As seen in Figure 11, melamine was at the bottom of the crucible while P25 was on the top of a cylinder put in the crucible. After a 4-h vapor deposition process, nanostructured g-C3N4/TiO2 composite was obtained.
2.3.2. The Role of g-C3N4 in Photocatalysis
2.4. MoS2 Modified TiO2
2.4.1. The Synthesis of MoS2/TiO2 Composites
- MoS2 as substrate. In this process, MoS2 are pre-prepared as substrate for the in-situ growth of TiO2. Hydrothermal method is widely used in which tetrabutyl titanate serves as titanate source [124,125]. Recently, another approach has been developed to synthesize MoS2@TiO2 composites. Ren et al. [126] reported TiO2-modified MoS2 nanosheet arrays by the ALD process, coating a thin layer of TiO2 on both the edge and basal planes of TiO2 (Figure 17). It provides a new insight for the combination of sites at the basal planes of TiO2.
- TiO2 composite as substrate. For coated MoS2/TiO2 composites, TiO2 are usually substrates. Liu et al. [127] reported a N-TiO2-x@MoS2 core-shell heterostructure composite. TBT and urea were used to prepare N-doped TiO2 microspheres (N-TiO2) with a smooth surface by hydrothermal method. Considering the growth of molybdenum sulfide on the TiO2 substrate, specific morphology and growth sites of TiO2 is needed. Sun et al. [128] took a targeted etching route to control the morphology of TiO2/MoS2 nanocomposites. Hollow microspheres structured TiO2/MoS2 showed a higher dye degradation activity due to a larger proportion of interface, compared to TiO2/MoS2 nanocomposites of yolk-shell structures. Other structures such as nanobelts and nanotubes have also been developed [129,130]. In addition to the morphology, the formation of a specific crystal structure of TiO2 as a substrate has also got attention to prepare high performance MoS2/TiO2 composites [130,131]. He et al. [130] reported a few-layered 1T-MoS2 coating on Si doped TiO2 nanotubes (MoS2/TiO2 NTs hybrids) through hydrothermal process. Because of the higher catalytic activity of 1T phase of MoS2 and Si doped TiO2, MoS2/TiO2 NTs hybrids nanocomposites exhibited excellent photocatalytic activity.
2.4.2. The Role of MoS2 in TiO2 Photocatalysis
3. Conclusions
Funding
Conflicts of Interest
References
- Lei, J.M.; Peng, Q.X.; Luo, S.P.; Liu, Y.; Zhan, S.Z.; Ni, C.L. A nickel complex, an efficient cocatalyst for both electrochemical and photochemical driven hydrogen production from water. Mol. Catal. 2018, 448, 10–17. [Google Scholar] [CrossRef]
- Wang, Z.; Jin, Z.; Yuan, H.; Wang, G.; Ma, B. Orderly-designed Ni2P nanoparticles on g-C3N4 and UiO-66 for efficient solar water splitting. J. Colloid Interface Sci. 2018, 532, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Vaiano, V.; Lara, M.A.; Iervolino, G.; Matarangolo, M.; Navio, J.A.; Hidalgo, M.C. Photocatalytic H2 production from glycerol aqueous solutions over fluorinated Pt-TiO2 with high {001} facet exposure. J. Photochem. Photobiol. A 2018, 365, 52–59. [Google Scholar] [CrossRef]
- Liu, Y.L.; Yang, C.L.; Wang, M.S.; Ma, X.G.; Yi, Y.G. Te-doped perovskite NaTaO3 as a promising photocatalytic material for hydrogen production from water splitting driven by visible light. Mater. Res. Bull. 2018, 107, 125–131. [Google Scholar] [CrossRef]
- Cai, Q.; Hu, J. Effect of UVA/LED/TiO2 photocatalysis treated sulfamethoxazole and trimethoprim containing wastewater on antibiotic resistance development in sequencing batch reactors. Water Res. 2018, 140, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Javed, H.; Zhang, D.; Kim, J.H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous electrospun fibers embedding TiO2 for adsorption and photocatalytic degradation of water pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Li, W.; Wan, W.; Zhang, R.; Lin, Y. W/Mo co-doped BiVO4 for photocatalytic treatment of polymer-containing wastewater in oilfield. Superlattice Microstruct. 2015, 82, 67–74. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Abou Saoud, W.; Assadi, A.A.; Guiza, M.; Bouzaza, A.; Aboussaoud, W.; Ouederni, A.; Soutrel, I.; Wolbert, D.; Rtimi, S. Study of synergetic effect, catalytic poisoning and regeneration using dielectric barrier discharge and photocatalysis in a continuous reactor: Abatement of pollutants in air mixture system. Appl. Catal. B Environ. 2017, 213, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ma, Z. Ag3VO4/AgI composites for photocatalytic degradation of dyes and tetracycline hydrochloride under visible light. Mater. Lett. 2018, 216, 216–219. [Google Scholar] [CrossRef]
- Huang, C.; Chen, H.; Zhao, L.; He, X.; Li, W.; Fang, W. Biogenic hierarchical MIL-125/TiO2@SiO2 derived from rice husk and enhanced photocatalytic properties for dye degradation. Photochem. Photobiol. 2018, 94, 512–520. [Google Scholar] [CrossRef]
- Kumar, M.; Mehta, A.; Mishra, A.; Singh, J.; Rawat, M.; Basu, S. Biosynthesis of tin oxide nanoparticles using psidium guajava leave extract for photocatalytic dye degradation under sunlight. Mater. Lett. 2018, 215, 121–124. [Google Scholar] [CrossRef]
- Dashairya, L.; Sharma, M.; Basu, S.; Saha, P. Enhanced dye degradation using hydrothermally synthesized nanostructured Sb2S3/rGO under visible light irradiation. J. Alloy Compd. 2018, 735, 234–245. [Google Scholar] [CrossRef]
- Xiao, L.; Lin, R.; Wang, J.; Cui, C.; Wang, J.; Li, Z. A novel hollow-hierarchical structured Bi2WO6 with enhanced photocatalytic activity for CO2 photoreduction. J. Colloid Interface Sci. 2018, 523, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Nie, N.; Zhang, L.; Fu, J.; Cheng, B.; Yu, J. Self-assembled hierarchical direct Z-scheme g-C3N4/ZnO microspheres with enhanced photocatalytic CO2, reduction performance. Appl. Surf. Sci. 2018, 441, 12–22. [Google Scholar] [CrossRef]
- Xia, P.; Zhu, B.; Yu, J.; Cao, S.; Jaroniec, M. Ultrathin nanosheet assemblies of graphitic carbon nitride for enhanced photocatalytic CO2 reduction. J. Mater. Chem. A 2017, 5, 3230–3238. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Li, R.; Guo, J.; Li, Y.; Zhu, J.; Xie, X. TiO2 supported on reed straw biochar as an adsorptive and photocatalytic composite for the efficient degradation of sulfamethoxazole in aqueous matrices. Chemosphere 2017, 185, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Geltmeyer, J.; Teixido, H.; Meire, M.; Van Acker, T.; Deventer, K.; Vanhaecke, F.; Van Hulle, S.; De Buysser, K.; De Clerck, K. TiO2 functionalized nanofibrous membranes for removal of organic (micro)pollutants from water. Sep. Purif. Technol. 2017, 179, 533–541. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Drunka, R.; Grabis, J.; Jankovica, D.; Krumina, A.; Rasmane, D. Microwave-assisted synthesis and photocatalytic properties of sulphur and platinum modified TiO2 nanofibers. IOP Conf. Sér. Mater. Sci. Eng. 2015, 77, 012010. [Google Scholar] [CrossRef]
- Dong, C.; Song, H.; Zhou, Y.; Dong, C.; Shen, B.; Yang, H.; Matsuoka, M.; Xing, M.; Zhang, J. Sulfur nanoparticles in situ growth on TiO2 mesoporous single crystals with enhanced solar light photocatalytic performance. RSC Adv. 2016, 6, 77863–77869. [Google Scholar] [CrossRef]
- Wang, S.; Pan, L.; Song, J.J.; Mi, W.; Zou, J.J.; Wang, L.; Zhang, X. Titanium-defected undoped anatase TiO2 with p-type conductivity, room-temperature ferromagnetism, and remarkable photocatalytic performance. J. Am. Chem. Soc. 2015, 137, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.R.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Z. Coupling plasmonic noble metal with TiO2, for efficient photocatalytic transfer hydrogenation: M/TiO2, (M = Au and Pt) for chemoselective transformation of cinnamaldehyde to cinnamyl alcohol under visible and 365 nm UV Light. Appl. Surf. Sci. 2018, 452, 279–285. [Google Scholar] [CrossRef]
- Matos, J.; Llano, B.; Montana, R.; Poon, P.S.; Hidalgo, M.C. Design of Ag/and Pt/TiO2-SiO2 nanomaterials for the photocatalytic degradation of phenol under solar irradiation. Environ. Sci. Pollut. Res. Int. 2018, 25, 18894–18913. [Google Scholar] [CrossRef] [PubMed]
- Roberto, F.; Orc, M.B.; Luisa, D.; Giuseppe, C.; Leonardo, P.; Salvatore, S. Au/TiO2-CeO2 catalysts for photocatalytic water splitting and VOCs oxidation reactions. Catalysts 2016, 6, 121. [Google Scholar] [CrossRef]
- Md Saad, S.K.; Ali Umar, A.; Ali Umar, M.I.; Tomitori, M.; Abd Rahman, M.Y.; Mat Salleh, M.; Oyama, M. Two-dimensional, hierarchical Ag-doped TiO2 nanocatalysts: Effect of the metal oxidation state on the photocatalytic properties. ACS Omega 2018, 3, 2579–2587. [Google Scholar] [CrossRef]
- Meryam, Z.; Benoit, S.; Mounira, M.; Ramzi, B.; Yu, W.; Wu, M.; Olivier, D.; Li, Y.; Su, B. ZnO quantum dots decorated 3DOM TiO2 nanocomposites: Symbiose of quantum size effects and photonic structure for highly enhanced photocatalytic degradation of organic pollutants. Appl. Catal. B Environ. 2016, 199, 187–198. [Google Scholar] [CrossRef]
- Luisa, M.P.; Sergio, M.-T.; Sónia, A.C.; Josephus, G.B.; José, L.F.; Adrián, M.T.; Silvab, J.L. Photocatalytic activity of functionalized nanodiamond-TiO2 composites towards water pollutants degradation under UV/Vis irradiation. Appl. Surf. Sci. 2018, 458, 839–848. [Google Scholar] [CrossRef]
- Prabhu, S.; Cindrella, L.; Kwon, O.J.; Mohanraju, K. Photoelectrochemical and photocatalytic activity of TiO2-WO3 heterostructures boosted by mutual interaction. Mater. Sci. Semicond. Process. 2018, 88, 10–19. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, Z.; Tan, C.; Zhang, H. Solution-processed two-dimensional MoS2 nanosheets: Preparation, hybridization, and applications. Angew. Chem. Int. Ed. Engl. 2016, 55, 8816–8838. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Hisatomi, T.; Kubota, J.; Domen, K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014, 43, 7520–7535. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Kwak, M.; Kim, Y.; Cheon, J. Recent advances in the solution-based preparation of two-dimensional layered transition metal chalcogenide nanostructures. Chem. Rev. 2018, 118, 6151–6188. [Google Scholar] [CrossRef] [PubMed]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Yuan, M.; Li, H.; Yang, H.; Zhou, Q.; Liu, H.; Lan, X.; Liu, M.; Wang, J.; Sargent, E.H.; et al. Graphdiyne: An efficient hole transporter for stable high-performance colloidal quantum dot solar cells. Adv. Funct. Mater. 2016, 26, 5284–5289. [Google Scholar] [CrossRef]
- Muller, G.A.; Cook, J.B.; Kim, H.S.; Tolbert, S.H.; Dunn, B. High performance pseudocapacitor based on 2D layered metal chalcogenide nanocrystals. Nano Lett. 2015, 15, 1911–1917. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. ACS Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wang, N.; Xue, Y.; Zuo, Z.; Liu, H.; Li, Y. Progress in research into 2D graphdiyne-based materials. Chem. Rev. 2018, 118, 7744–7803. [Google Scholar] [CrossRef]
- Fiori, G.; Francesco, B.; Iannaccone, G.; Palacios, T.; Neumaier, D.; Seabaugh, A.; Banerjee, S.K.; Colombo, L. Electronics based on two-dimensional materials. Nat. Nanotechnol. 2014, 9, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Tao, C.; Zhang, X.; Zhao, W.; Zhang, H. Solution-processed two-dimensional metal dichalcogenide-based nanomaterials for energy storage and conversion. Adv. Mater. 2016, 28, 6167–6196. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotechnol. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Hu, G.; Gao, H.; Hai, L. Application of graphene as filler to improve thermal transport property of epoxy resin for thermal interface materials. Int. J. Heat Mass Transf. 2015, 85, 420–429. [Google Scholar] [CrossRef]
- Tang, B.; Guoxin, H.; Gao, H. Raman spectroscopic characterization of graphene. Appl. Spectrosc. Rev. 2010, 45, 369–407. [Google Scholar] [CrossRef]
- Wang, Q.; Arash, B. A review on applications of carbon nanotubes and graphenes as nano-resonator sensors. Comput. Mater. Sci. 2014, 82, 350–360. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.-J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Z.; Li, N.; Pu, Y.; Wang, B.; Zhang, T.; Tao, J. Advanced review of graphene-based nanomaterials in drug delivery systems: Synthesis, modification, toxicity and application. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1363–1375. [Google Scholar] [CrossRef] [PubMed]
- Chabot, V.; Higgins, D.; Yu, A.; Xiao, X.; Chen, Z.; Zhang, J. A review of graphene and graphene oxide sponge: Material synthesis and applications to energy and the environment. Energy Environ. Sci. 2014, 7, 1564–1596. [Google Scholar] [CrossRef]
- Hu, G.; Tang, B. Photocatalytic mechanism of graphene/titanate nanotubes photocatalyst under visible-light irradiation. Mater. Chem. Phys. 2013, 138, 608–614. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, X.; Cao, Y.; Xiang, J.; Gao, H. Enhanced photocatalytic activities of low-bandgap TiO2-reduced graphene oxide nanocomposites. J. Nanopart. Res. 2017, 19. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, H.; Guo, L.H.; Zhao, L. Two-dimensional interface engineering of a Titania-graphene nanosheet composite for improved photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 13035–13041. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Y.; Nie, Y.; Kavadiya, S.; Soundappan, T.; Biswas, P. N-doped reduced graphene oxide promoted nano TiO2 as a bifunctional adsorbent/photocatalyst for CO2 photoreduction: Effect of N species. Chem. Eng. J. 2017, 316, 449–460. [Google Scholar] [CrossRef]
- Zhu, M.; Muhammad, Y.; Hu, P.; Wang, B.; Wu, Y.; Sun, X.; Tong, Z.; Zhao, Z. Enhanced interfacial contact of dopamine bridged melamine-graphene/TiO2, nano-capsules for efficient photocatalytic degradation of gaseous formaldehyde. Appl. Catal. B Environ. 2018, 232, 182–193. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Jaroniec, M. Graphene-based semiconductor photocatalysts. Chem. Soc. Rev. 2012, 41, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Hummers, J.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Bai, H.; Li, C.; Shi, G. Functional composite materials based on chemically converted graphene. Adv. Mater. 2011, 23, 1089–1115. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Zargari, S.; Sadat Shojaei, Z. Photoelectrochemical investigation of TiO2-graphene nanocomposites. In Proceedings of the International Electronic Conference on Synthetic Organic Chemistry, Tehran, Iran, 18 November 2014; pp. 1–10. [Google Scholar]
- Pogacean, F.; Rosu, M.C.; Coros, M. Graphene/TiO2-Ag based composites used as sensitive electrode materials for amaranth electrochemical detection and degradation. J. Electrochem. Soc. 2018, 165, B3054–B3059. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Chemical functionalization of graphene and its applications. Prog. Mater Sci. 2012, 57, 1061–1105. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, Z.; Zhao, X.S. Graphene–metal–oxide composites for the degradation of dyes under visible light irradiation. J. Mater. Chem. 2011, 21, 3634–3640. [Google Scholar] [CrossRef]
- Fan, W.; Lai, Q.; Zhang, Q.; Wang, Y. Nanocomposites of TiO2 and reduced graphene oxide as efficient photocatalysts for hydrogen evolution. J. Phys. Chem. C 2011, 115, 10694–10701. [Google Scholar] [CrossRef]
- Fu, C.; Chen, T.; Qin, W.; Lu, T.; Sun, Z.; Xie, X.; Pan, L. Scalable synthesis and superior performance of TiO2-reduced graphene oxide composite anode for sodium-ion batteries. Ionics 2015, 22, 555–562. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Mao, H.; Huang, Y.; Ding, Q.; Pang, X. Hydroxyapatite/gelatin functionalized graphene oxide composite coatings deposited on TiO2 nanotube by electrochemical deposition for biomedical applications. Appl. Surf. Sci. 2015, 329, 76–82. [Google Scholar] [CrossRef]
- Anjusree, G.S.; Nair, A.S.; Nair, S.V.; Vadukumpully, S. One-pot hydrothermal synthesis of TiO2/graphene nanocomposites for enhanced visible light photocatalysis and photovoltaics. RSC Adv. 2013, 3, 12933–12938. [Google Scholar] [CrossRef]
- Shi, M.; Shen, J.; Ma, H.; Li, Z.; Lu, X.; Li, N.; Ye, M. Preparation of graphene–TiO2 composite by hydrothermal method from peroxotitanium acid and its photocatalytic properties. Colloids Surf. Physicochem. Eng. Aspects 2012, 405, 30–37. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Li, H.P.; Cui, X.L.; Lin, Y. Graphene/TiO2 nanocomposites: Synthesis, characterization and application in hydrogen evolution from water photocatalytic splitting. J. Mater. Chem. 2010, 20, 2801–2806. [Google Scholar] [CrossRef]
- Farhangi, N.; Chowdhury, R.R.; Medina-Gonzalez, Y.; Ray, M.B.; Charpentier, P.A. Visible light active Fe doped TiO2, nanowires grown on graphene using supercritical CO2. Appl. Catal. B Environ. 2011, 110, 25–32. [Google Scholar] [CrossRef]
- Peng, R.; Liang, L.; Hood, Z.D.; Boulesbaa, A.; Puretzky, A.; Ievlev, A.V.; Come, J.; Ovchinnikova, O.S.; Wang, H.; Ma, C.; et al. In-plane heterojunctions enable multiphasic two-dimensional (2D) MoS2 nanosheets as efficient photocatalysts for hydrogen evolution from water reduction. ACS Catal. 2016, 6, 6723–6729. [Google Scholar] [CrossRef]
- Zhu, Z.; Han, Q.; Yu, D.; Sun, J.; Liu, B. A novel p-n heterojunction of BiVO4/TiO2/GO composite for enhanced visible-light-driven photocatalytic activity. Mater. Lett. 2017, 209, 379–383. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Liu, H.; Guo, Y.; Li, Y.; Zhu, D. Architecture of graphdiyne nanoscale films. Chem. Commun. 2010, 46, 3256–3258. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, R.; Sakamoto, R.; Hoshiko, K.; Sasaki, S.; Masunaga, H.; Nagashio, K.; Nishihara, H. Crystalline graphdiyne nanosheets produced at a gas/liquid or liquid/liquid interface. J. Am. Chem. Soc. 2017, 139, 3145–3152. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.P.; Wang, H.J.; Tang, S.F.; Lu, X.L.; Shu, M.; Si, R.; Lu, T.B. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. Engl. 2018, 57, 9382–9386. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Jin, Q.; Wei, Y.; Yu, R.; Zheng, B.; Huang, L.; Zhang, Y.; Wang, L.; Zhang, H.; Gao, M.; et al. Few-layer graphdiyne nanosheets applied for multiplexed real-time DNA detection. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wang, N.; Cui, Z.; Du, H.; Fu, L.; Huang, C.; Yang, Z.; Shen, X.; Yi, Y.; Tu, Z.; et al. Hydrogen substituted graphdiyne as carbon-rich flexible electrode for lithium and sodium ion batteries. Nat. Commun. 2017, 8, 1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, K.; Wang, N.; He, J.; Yang, Z.; Shen, X.; Huang, C. Preparation of 3D architecture graphdiyne nanosheets for high-performance sodium-ion batteries and capacitors. ACS Appl. Mater. Interfaces 2017, 9, 40604–40613. [Google Scholar] [CrossRef] [PubMed]
- Diederich, F. Carbon scaffolding-building acetylenic all-carbon and carbon-rich compounds. Nature 1994, 369, 199–207. [Google Scholar] [CrossRef]
- Xue, Y.; Li, Y.; Zhang, J.; Liu, Z.; Zhao, Y. 2D graphdiyne materials: Challenges and opportunities in energy field. Sci. China Chem. 2018, 61, 765–786. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y.; Wen, H.; Tang, Z.; Zhao, H.; Li, Y.; Wang, D. Photocatalytic properties of graphdiyne and graphene modified TiO2: From theory to experiment. ACS Nano 2013, 7, 1504–1512. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yi, L.; Halpert, J.E.; Lai, X.; Liu, Y.; Cao, H.; Yu, R.; Wang, D.; Li, Y. A novel and highly efficient photocatalyst based on P25-graphdiyne nanocomposite. Small 2012, 8, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Li, Y.; Zuo, Z.; Liu, H.; Huang, C.; Li, Y. Synthesis and properties of 2D carbon-graphdiyne. Acc. Chem. Res. 2017, 50, 2470–2478. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhao, Y.; Chen, Y.; Feng, Y.; Zhu, M.; Ju, C.; Zhang, B.; Liu, H.; Xu, J. Graphdiyne-hybridized N-doped TiO2 nanosheets for enhanced visible light photocatalytic activity. J. Mater. Sci. 2018, 53, 8921–8932. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Qian, X.; Liu, H.; Lin, H.; Chen, N.; Li, Y. Construction of tubular molecule aggregations of graphdiyne for highly efficient field emission. J. Phys. Chem. C 2011, 115, 2611–2615. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, X.; Liu, R.; Xie, Z.; Yang, J.; Zhang, S.; Zhang, G.; Liu, H.; Li, Y.; Zhang, J.; et al. Synthesis of graphdiyne nanowalls using acetylenic coupling reaction. J. Am. Chem. Soc. 2015, 137, 7596–7599. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, L.; Liu, H.; Li, Y. Graphdiyne and graphyne: From theoretical predictions to practical construction. Chem. Soc. Rev. 2014, 43, 2572–2586. [Google Scholar] [CrossRef] [PubMed]
- Thangavel, S.; Krishnamoorthy, K.; Krishnaswamy, V.; Raju, N.; Kim, S.J.; Venugopal, G. Graphdiyne–ZnO nanohybrids as an advanced photocatalytic material. J. Phys. Chem. C 2015, 119, 22057–22065. [Google Scholar] [CrossRef]
- Long, M.; Tang, L.; Wang, D.; Li, Y.; Shuai, Z. Electronic structure and carrier mobility in graphdiyne sheet and nanoribbons: Theoretical predictions. ACS Nano 2011, 5, 2593–2600. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Yu, P.; Wang, Y.; Han, G.; Liu, H.; Yi, Y.; Li, Y.; Mao, L. Graphdiyne oxides as excellent substrate for electroless deposition of pd clusters with high catalytic activity. J. Am. Chem. Soc. 2015, 137, 5260–5263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhu, M.; Chen, P.; Li, Y.; Liu, H.; Li, Y.; Liu, M. Pristine graphdiyne-hybridized photocatalysts using graphene oxide as a dual-functional coupling reagent. Phys. Chem. Chem. Phys. 2015, 17, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, M.; Zhu, Y. Photocatalytic enhancement of hybrid C3N4/TiO2 prepared via ball milling method. Phys. Chem. Chem. Phys. 2015, 17, 3647–3652. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wang, J.; Zou, Z.; Han, X. Graphitic-C3N4-hybridized TiO2 nanosheets with reactive {001} facets to enhance the UV- and visible-light photocatalytic activity. J. Hazard. Mater. 2014, 268, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Y.; Wu, X.; Lee, P.H.; Shih, K. Fabrication of heterostructured g-C3N4/Ag-TiO2 hybrid photocatalyst with enhanced performance in photocatalytic conversion of CO2 under simulated sunlight irradiation. Appl. Surf. Sci. 2017, 402, 198–207. [Google Scholar] [CrossRef]
- Li, K.; Huang, Z.; Zeng, X.; Huang, B.; Gao, S.; Lu, J. Synergetic effect of Ti3+ and oxygen doping on enhancing photoelectrochemical and photocatalytic properties of TiO2/g-C3N4 heterojunctions. ACS Appl. Mater. Interfaces 2017, 9, 11577–11586. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhou, Y.; Bao, J.; Fang, J.; Zhao, S.; Zhang, Y.; Sheng, X.; Chen, W. Structure regulation of ZnS@g-C3N4/TiO2 nanospheres for efficient photocatalytic H2 production under visible-light irradiation. Chem. Eng. J. 2018, 346, 226–237. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Ma, J.; Wang, X.; Wang, J.; Xia, S.; Zhao, J. Removal of microcystis aeruginosa and microcystin-LR using a graphitic-C3N4/TiO2 floating photocatalyst under visible light irradiation. Chem. Eng. J. 2018, 348, 380–388. [Google Scholar] [CrossRef]
- Ricci, P.C.; Laidani, N.; Chiriu, D.; Salis, M.; Carbonaro, C.M.; Corpino, R. ALD growth of metal oxide on carbon nitride polymorphs. Appl. Surf. Sci. 2018, 456, 83–94. [Google Scholar] [CrossRef]
- Ren, B.; Wang, T.; Qu, G.; Deng, F.; Liang, D.; Yang, W.; Liu, M. In situ synthesis of g-C3N4/TiO2 heterojunction nanocomposites as a highly active photocatalyst for the degradation of orange ii under visible light irradiation. Environ. Sci. Pollut. Res. 2018, 25, 19122–19133. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, F.; Bo, C.; Cheng, K.; Wang, J.; Zhang, J.; Song, H. Promotion of phenol photodecomposition and the corresponding decomposition mechanism over g-C3N4/TiO2 nanocomposites. Appl. Surf. Sci. 2018, 453, 320–329. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Guan, J.; Liu, C.; Zhao, X.; Zhu, Z.; Ma, C.; Huo, P.; Li, C.; Yan, Y. Improved photoelectric performance via fabricated heterojunction g-C3N4/TiO2/HNTS loaded photocatalysts for photodegradation of ciprofloxacin. J. Ind. Eng. Chem. 2018, 64, 206–218. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Elbanna, O.; Fujitsuka, M.; Majima, T. g-C3N4/TiO2 mesocrystals composite for H2 evolution under visible-light irradiation and its charge carrier dynamics. ACS Appl. Mater. Interfaces 2017, 9, 34844–34854. [Google Scholar] [CrossRef] [PubMed]
- Guo, N.; Zeng, Y.; Li, H.; Xu, X.; Yu, H.; Han, X. Novel mesoporous TiO2@g-C3N4 hollow core@shell heterojunction with enhanced photocatalytic activity for water treatment and H2 production under simulated sunlight. J. Hazard. Mater. 2018, 353, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Li, F.; Liu, Y.; Hong, Y.; Liu, P.; Ni, L. Construction of TiO2 hollow nanosphere/g-C3N4 composites with superior visible-light photocatalytic activity and mechanism insight. J. Ind. Eng. Chem. 2016, 41, 130–140. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of heterostructured g-C3N4/Ag-TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, S.; Sun, X.; Xu, X.; Gao, B. Unique bar-like sulfur-doped g-C3N4/TiO2 nanocomposite: Excellent visible light driven photocatalytic activity and mechanism study. Appl. Surf. Sci. 2018, 436, 873–881. [Google Scholar] [CrossRef]
- Cheng, L.; Liu, J.; Gu, X.; Gong, H.; Shi, X.; Liu, T.; Wang, C.; Wang, X.; Liu, G.; Xing, H.; et al. Pegylated WS2 nanosheets as a multifunctional theranostic agent for in vivo dual-modal CT/photoacoustic imaging guided photothermal therapy. Adv. Mater. 2014, 26, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Xiao, X.; Li, Y.; Zhang, W. Enhanced photocatalytic activity of TiO2 nanoparticles using WS2/g-C3N4 hybrid as co-catalyst. Trans. Nonferrous Met. Soc. China 2017, 27, 1117–1126. [Google Scholar] [CrossRef]
- He, H.Y. Solvothermal synthesis and photocatalytic activity of s-doped TiO2 and TiS2 powders. Res. Chem. Intermed. 2010, 36, 155–161. [Google Scholar] [CrossRef]
- Shen, M.; Yan, Z.; Yang, L.; Du, P.; Zhang, J.; Xiang, B. MoS2 nanosheet/TiO2 nanowire hybrid nanostructures for enhanced visible-light photocatalytic activities. Chem. Commun. 2014, 50, 15447–15449. [Google Scholar] [CrossRef] [PubMed]
- Hai, X.; Chang, K.; Pang, H.; Li, M.; Li, P.; Liu, H.; Shi, L.; Ye, J. Engineering the edges of MoS2 (WS2) crystals for direct exfoliation into monolayers in polar micromolecular solvents. J. Am. Chem. Soc. 2016, 138, 14962–14969. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zang, C.; Huang, Z.; Zhang, H.; Ren, L.; Qi, X.; Zhong, J. Enhanced photocatalytic activities of three-dimensional graphene-based aerogel embedding TiO2 nanoparticles and loading MoS2 nanosheets as co-catalyst. Int. J. Hydrogen Energy 2014, 39, 19502–19512. [Google Scholar] [CrossRef]
- Nimbalkar, D.B.; Lo, H.-H.; Ramacharyulu, P.V.R.K.; Ke, S.C. Improved photocatalytic activity of rGO/MoS2 nanosheets decorated on TiO2 nanoparticles. RSC Adv. 2016, 6, 31661–31667. [Google Scholar] [CrossRef]
- Wang, D.; Xu, Y.; Sun, F.; Zhang, Q.; Wang, P.; Wang, X. Enhanced photocatalytic activity of TiO2 under sunlight by MoS2 nanodots modification. Appl. Surf. Sci. 2016, 377, 221–227. [Google Scholar] [CrossRef]
- Pi, Y.; Li, Z.; Xu, D.; Liu, J.; Li, Y.; Zhang, F.; Zhang, G.; Peng, W.; Fan, X. 1T-phase MoS2 nanosheets on TiO2 nanorod arrays: 3D photoanode with extraordinary catalytic performance. ACS Sustain. Chem. Eng. 2017, 5, 5175–5182. [Google Scholar] [CrossRef]
- Yu, Y.; Wan, J.; Yang, Z.; Hu, Z. Preparation of the MoS2/TiO2/HMFS ternary composite hollow microfibres with enhanced photocatalytic performance under visible light. J. Colloid Interface Sci. 2017, 502, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-J.; Ye, Z.J.; Lu, H.W.; Hu, B.; Li, Y.-H.; Chen, D.Q.; Zhong, J.S.; Yu, Z.T.; Zou, Z.G. Constructing anatase TiO2 nanosheets with exposed (001) facets/layered MoS2 two-dimensional nanojunctions for enhanced solar hydrogen generation. ACS Catal. 2015, 6, 532–541. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, H.; Wang, W.; Zhang, Y.; Li, Z.; Wu, X.; Yu, X.; Zhou, W. Fabrication of 3D mesoporous black TiO2/MoS2/TiO2 nanosheets for visible-light-driven photocatalysis. ChemSusChem 2016, 9, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lin, J.; Fu, W.; Wang, X.; Wang, H.; Zeng, Q.; Gu, Q.; Li, Y.; Yan, C.; Tay, B.K.; et al. MoS2/TiO2 edge-on heterostructure for efficient photocatalytic hydrogen evolution. Adv. Funct. Mater. 2016, 6, 1600464. [Google Scholar] [CrossRef]
- Van Haandel, L.; Geus, J.W.; Weber, T. Direct synthesis of TiO2-supported MoS2 nanoparticles by reductive coprecipitation. ChemCatChem 2016, 8, 1367–1372. [Google Scholar] [CrossRef]
- Ren, X.; Qi, X.; Shen, Y.; Xiao, S.; Xu, G.; Zhang, Z.; Huang, Z.; Zhong, J. 2D co-catalytic MoS2 nanosheets embedded with 1D TiO2 nanoparticles for enhancing photocatalytic activity. J. Phys. D Appl. Phys. 2016, 49, 315304. [Google Scholar] [CrossRef]
- Bai, S.; Wang, L.; Chen, X.; Du, J.; Xiong, Y. Chemically exfoliated metallic MoS2 nanosheets: A promising supporting co-catalyst for enhancing the photocatalytic performance of TiO2 nanocrystals. Nano Res. 2014, 8, 175–183. [Google Scholar] [CrossRef]
- Kim, Y.; Jackson, D.H.K.; Lee, D.; Choi, M.; Kim, T.W.; Jeong, S.-Y.; Chae, H.J.; Kim, H.W.; Park, N.; Chang, H.; et al. In situ electrochemical activation of atomic layer deposition coated MoS2 basal planes for efficient hydrogen evolution reaction. Adv. Funct. Mater. 2017, 27, 1701825. [Google Scholar] [CrossRef]
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black n- TiO2−x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2017, 201, 119–127. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, H.; Wang, C.; Wu, Q.; Wang, X.; Yang, M. Morphology-controlled synthesis of TiO2/MoS2 nanocomposites with enhanced visible-light photocatalytic activity. Inorg. Chem. Front. 2018, 5, 145–152. [Google Scholar] [CrossRef]
- Pu, S.; Long, D.; Wang, M.Q.; Bao, S.J.; Liu, Z.; Yang, F.; Wang, H.; Zeng, Y. Design, synthesis and photodegradation ammonia properties of MoS2@TiO2 encapsulated carbon coaxial nanobelts. Mater. Lett. 2017, 209, 56–59. [Google Scholar] [CrossRef]
- He, H.Y. Efficient hydrogen evolution activity of 1T- MoS2/Si-doped TiO2 nanotube hybrids. Int. J. Hydrogen Energy 2017, 42, 20739–20748. [Google Scholar] [CrossRef]
- Han, H.; Kim, K.M.; Lee, C.W.; Lee, C.S.; Pawar, R.C.; Jones, J.L.; Hong, Y.R.; Ryu, J.H.; Song, T.; Kang, S.H.; et al. Few-layered metallic 1T-MoS2/TiO2 with exposed (001) facets: Two-dimensional nanocomposites for enhanced photocatalytic activities. Phys. Chem. Chem. Phys. 2017, 19, 28207–28215. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Kang, B.; Jeon, M.; Huffman, C.; Jeon, J.; Lee, S.; Han, W.; Lee, J.; Lee, S.; Yeom, G.; et al. Controlled layer-by-layer etching of MoS2. ACS Appl. Mater. Interfaces 2015, 7, 15892–15897. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Xie, Y.; Zhou, J.; Li, Y.; Yu, Y.; Ren, Z. Robust and selective electrochemical reduction of CO2: The case of integrated 3D TiO2@MoS2 architectures and Ti–S bonding effects. J. Mater. Chem. A 2018, 6, 4706–4713. [Google Scholar] [CrossRef]
- Jaramillo, T.F.; Jorgensen, K.P.; Bonde, J.; Nielsen, J.H.; Horch, S.; Chorkendorff, I. Identification of active edge sites for electrochemical H2 evolution from MoS2 nanocatalysts. Science 2007, 317, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Yu, J.; Jaroniec, M. Synergetic effect of MoS2 and graphene as cocatalysts for enhanced photocatalytic H2 production activity of TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 6575–6578. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhao, H.; Tian, J.; Gao, J.; Li, Y.; Cui, H. Synthesis of few-layer MoS2 nanosheets-coated TiO2 nanosheets on graphite fibers for enhanced photocatalytic properties. Sol. Energy Mater. Sol. Cells 2017, 172, 108–116. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, P.; Fan, J.; Hu, J.; Shao, G. Constructing 2D layered MoS2 nanosheets-modified z-scheme TiO2/WO3 nanofibers ternary nanojunction with enhanced photocatalytic activity. Appl. Surf. Sci. 2018, 430, 466–474. [Google Scholar] [CrossRef]






















| Primary Process | Characteristic Times | |
|---|---|---|
| charge-carrier generation | TiO2 + hv → hVB+ + ecb− | (fs) |
| charge-carrier trapping | hVB+ + >TiIVOH → {>TiIVOH}•+ | fast (10 ns) |
| ecb− + >TiIVOH → {>TiIIIOH} | shallow trap (100 ps) (dynamic equilibrium) | |
| ecb− + >TiIV → >TiIII | deep trap (10 ns) (irreversible) | |
| charge-carrier recombination | ecb− + {>TiIVOH}•+ → >TiIVOH | slow (100 ns) |
| hVB+ + {>TiIIIOH} → TiIVOH | fast (10 ns) | |
| interfacial charge transfer | {>TiIVOH}•+ + Red → >TiIVOH + Red•+ | slow (100 ns) |
| etr− + Ox → TiIVOH + Ox•− | very slow (ms) | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Dong, Y.; Feng, Y.; Xu, J. Compositing Two-Dimensional Materials with TiO2 for Photocatalysis. Catalysts 2018, 8, 590. https://doi.org/10.3390/catal8120590
Ren Y, Dong Y, Feng Y, Xu J. Compositing Two-Dimensional Materials with TiO2 for Photocatalysis. Catalysts. 2018; 8(12):590. https://doi.org/10.3390/catal8120590
Chicago/Turabian StyleRen, Yu, Yuze Dong, Yaqing Feng, and Jialiang Xu. 2018. "Compositing Two-Dimensional Materials with TiO2 for Photocatalysis" Catalysts 8, no. 12: 590. https://doi.org/10.3390/catal8120590
APA StyleRen, Y., Dong, Y., Feng, Y., & Xu, J. (2018). Compositing Two-Dimensional Materials with TiO2 for Photocatalysis. Catalysts, 8(12), 590. https://doi.org/10.3390/catal8120590