Catalytic Efficiency of Basidiomycete Laccases: Redox Potential versus Substrate-Binding Pocket Structure
Abstract
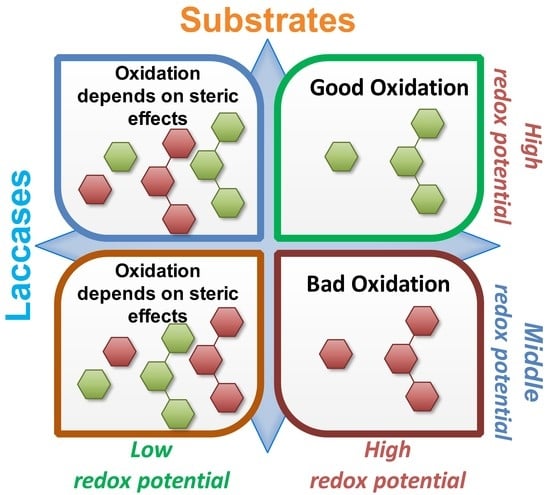
:1. Introduction
2. Results and Discussion
2.1. Substrate Specificities of Laccases toward Monophenolic Compounds and Phenolic Dyes
2.2. Kinetics of Oxidation of Lignans by Laccases
3. Materials and Methods
3.1. Fungal Strains
3.2. Culturing Conditions and Enzyme Purification
3.3. Biochemical Assays
3.4. Enzyme Structure Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial Sources, Production, Purification, and Potential Biotechnological Applications. Enzyme Res. 2011, 2011, 1–11. [Google Scholar] [CrossRef]
- Rivera-Hoyos, C.M.; Morales-Alvarez, E.D.; Poutou-Pinales, R.A.; Pedroza-Rodriguez, A.M.; Rodriguez-Vazquez, R.; Delgado-Boada, J.M. Fungal laccases. Fungal Biol. Rev. 2013, 27, 67–82. [Google Scholar] [CrossRef]
- Reiss, R.; Ihssen, J.; Richter, M.; Eichhorn, E.; Schilling, B.; Thöny-Meyer, L. Laccase versus Laccase-Like Multi-Copper Oxidase: A Comparative Study of Similar Enzymes with Diverse Substrate Spectra. PLoS ONE 2013, 8, e65633. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P. Fungal laccases—Occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Frasconi, M.; Favero, G.; Boer, H.; Koivula, A.; Mazzei, F. Kinetic and biochemical properties of high and low redox potential laccases from fungal and plant origin. Biochim. Biophys. Acta 2010, 1804, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, M.; D’Annibale, A.; Galli, C. An assessment of the relative contributions of redox and steric issues to laccase specificity towards putative substrates. Org. Biomol. Chem. 2008, 6, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Galli, C.; Gentili, P.; Jolivalt, C. How is the reactivity of laccase affected by single-point mutations? Engineering laccase for improved activity towards sterically demanding substrates. Appl. Microbiol. Biotechnol. 2011, 91, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Rebrikov, D.V.; Stepanova, E.V.; Koroleva, O.V.; Budarina, Z.I.; Zakharova, M.V.; Yurkova, T.V.; Solonin, A.S.; Belova, O.V.; Pozhidaeva, Z.A.; et al. Laccase of the lignolytic fungus Trametes hirsuta: Purification and characterization of the enzyme and cloning and primary structure of the gene. Appl. Biochem. Microbiol. 2007, 43, 365. [Google Scholar] [CrossRef]
- Glazunova, O.A.; Polyakov, K.M.; Fedorova, T.V.; Dorovatovskii, P.V.; Koroleva, O.V. Elucidation of the crystal structure of Coriolopsis caperata laccase: Restoration of the structure and activity of the native enzyme from the T2-depleted form by copper ions. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 854–861. [Google Scholar] [CrossRef] [PubMed]
- Glazunova, O.A.; Fedorova, T.V.; Maloshenok, L.G.; Shakhova, N.V.; Polyakov, K.M.; Koroleva, O.V. Purification and characterization of novel laccase from basidiomycete Antrodiella faginea 1998. FEBS J. 2013, 280, 124. [Google Scholar]
- Polyakov, K.M.; Fedorova, T.V.; Stepanova, E.V.; Cherkashin, E.A.; Kurzeev, S.A.; Strokopytov, B.V.; Lamzin, V.S.; Koroleva, O.V. Structure of native laccase from Trametes hirsuta at 1.8 A resolution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Glazunova, O.A.; Polyakov, K.M.; Moiseenko, K.V.; Kurzeev, S.A.; Fedorova, T.V. Structure-function study of two new middle-redox potential laccases from basidiomycetes Antrodiella faginea and Steccherinum murashkinskyi. 2018, in press. [Google Scholar]
- González, M.D.; Vidal, T.; Tzanov, T. Electrochemical study of phenolic compounds as enhancers in laccase-catalyzed oxidative reactions. Electroanalysis 2009, 21, 2249–2257. [Google Scholar] [CrossRef]
- Yakovleva, K.E.; Kurzeev, S.A.; Stepanova, E.V.; Fedorova, T.V.; Kuznetsov, B.A.; Koroleva, O.V. Characterization of Plant Phenolic Compounds by Cyclic Voltammetry. Prikl. Biokhim. Mikrobiol. 2007, 43, 730–739. [Google Scholar] [CrossRef]
- Suatoni, J.C.; Snyder, R.E.; Clark, R.O. Voltammetric Studies of Phenol and Aniline Ring Substitution. Anal. Chem. 1961, 33, 1894–1897. [Google Scholar] [CrossRef]
- Meyer, H.W.; Treadwell, W.D. Über die Redoxpotentiale von einigen Polyoxyanthrachinonen und Küpenfarbstoffen. Helv. Chim. Acta 1952, 35, 1444–1460. [Google Scholar] [CrossRef]
- Sun, J.; Hu, Y.Y.; Hou, B. Electrochemical characteriztion of the bioanode during simultaneous azo dye decolorization and bioelectricity generation in an air-cathode single chambered microbial fuel cell. Electrochim. Acta 2011, 56, 6874–6879. [Google Scholar] [CrossRef]
- Maijala, P.; Mattinen, M.-L.; Nousiainen, P.; Kontro, J.; Asikkala, J.; Sipilä, J.; Viikari, L. Action of fungal laccases on lignin model compounds in organic solvents. J. Mol. Catal. B Enzym. 2012, 76, 59–67. [Google Scholar] [CrossRef]
- Mattinen, M.L.; Struijs, K.; Suortti, T.; Mattila, I.; Kruus, K.; Willför, S.; Tamminen, T.; Vincken, J.P. Modification of lignans by Trametes hirsuta laccase. BioResources 2009, 4, 482–496. [Google Scholar]
- Bertrand, T.; Jolivalt, C.; Briozzo, P.; Caminade, E.; Joly, N.; Madzak, C.; Mougin, C. Crystal structure of a four-copper laccase complexed with an arylamine: Insights into substrate recognition and correlation with kinetics. Biochemistry 2002, 41, 7325–7333. [Google Scholar] [CrossRef] [PubMed]
- Kallio, J.; Auer, S.; Jänis, J.; Andberg, M.; Kruus, K.; Rouvinen, J.; Koivula, A.; Hakulinen, N. Structure–function studies of a Melanocarpus albomyces laccase suggest a pathway for oxidation of phenolic compounds. J. Mol. Biol. 2009, 392, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, T.V.; Shakhova, N.V.; Klein, O.I.; Glazunova, O.A.; Maloshenok, L.G.; Kulikova, N.A.; Psurtseva, N.V.; Koroleva, O.V. Comparative analysis of the ligninolytic potential of basidiomycetes belonging to different taxonomic and ecological groups. Appl. Biochem. Microbiol. 2013, 49, 570–580. [Google Scholar] [CrossRef]
- Parthasarathy, S.; Murthy, M.R.N. Analysis of temperature factor distribution in high-resolution protein structures. Protein Sci. 1997, 6, 2561–2567. [Google Scholar] [CrossRef] [PubMed]
- McNicholas, S.; Potterton, E.; Wilson, K.S.; Noble, M.E.M. Presenting your structures: The CCP4mg molecular-graphics software. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 386–394. [Google Scholar] [CrossRef] [PubMed]



| SECO | ETO | SDG | MANG | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| КМ (µM) | Vmax (µM·s−1) | Vmax/K (×104 s−1) | КМ (µM) | Vmax (µM·s−1) | Vmax/K (×104 s−1) | КМ (µM) | Vmax (µM·s−1) | Vmax/K (×104 s−1) | КМ (µM) | Vmax (µM·s−1) | Vmax/K (×104 s−1) | |
| ThL | 31 ± 6 | 1.75 ± 0.08 | 565 | 54 ± 15 | 1.52 ± 0.13 | 282 | 108 ± 19 | 1.21 ± 0.07 | 112 | 112 ± 33 | 0.57 ± 0.06 | 51 |
| CcL | 60 ± 7 | 1.68 ± 0.06 | 280 | 74 ± 11 | 1.83 ± 0.09 | 247 | 190 ± 15 | 1.60 ± 0.05 | 84 | 229 ± 55 | 0.64 ± 0.07 | 28 |
| AfL | 124 ± 6 | 2.04 ± 0.04 | 165 | 113 ± 16 | 1.80 ± 0.10 | 160 | 370 ± 35 | 1.23 ± 0.06 | 33 | 100 ± 15 | 1.06 ± 0.05 | 106 |
| SmL | 172 ± 26 | 1.76 ± 0.12 | 102 | 91 ± 64 | 0.30 ± 0.08 | 33 | 135 ± 29 | 0.34 ± 0.03 | 25 | -- | <0.05 | < 10 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glazunova, O.A.; Trushkin, N.A.; Moiseenko, K.V.; Filimonov, I.S.; Fedorova, T.V. Catalytic Efficiency of Basidiomycete Laccases: Redox Potential versus Substrate-Binding Pocket Structure. Catalysts 2018, 8, 152. https://doi.org/10.3390/catal8040152
Glazunova OA, Trushkin NA, Moiseenko KV, Filimonov IS, Fedorova TV. Catalytic Efficiency of Basidiomycete Laccases: Redox Potential versus Substrate-Binding Pocket Structure. Catalysts. 2018; 8(4):152. https://doi.org/10.3390/catal8040152
Chicago/Turabian StyleGlazunova, Olga A., Nikita A. Trushkin, Konstantin V. Moiseenko, Ivan S. Filimonov, and Tatyana V. Fedorova. 2018. "Catalytic Efficiency of Basidiomycete Laccases: Redox Potential versus Substrate-Binding Pocket Structure" Catalysts 8, no. 4: 152. https://doi.org/10.3390/catal8040152
APA StyleGlazunova, O. A., Trushkin, N. A., Moiseenko, K. V., Filimonov, I. S., & Fedorova, T. V. (2018). Catalytic Efficiency of Basidiomycete Laccases: Redox Potential versus Substrate-Binding Pocket Structure. Catalysts, 8(4), 152. https://doi.org/10.3390/catal8040152