An Improved Method to Encapsulate Laccase from Trametes versicolor with Enhanced Stability and Catalytic Activity
Abstract
:1. Introduction
2. Results
2.1. The Effect of Immobilization Protocols
2.2. FTIR
2.3. The Effect of Alginate Concentration
2.4. pH Stability
2.5. Thermal Stability
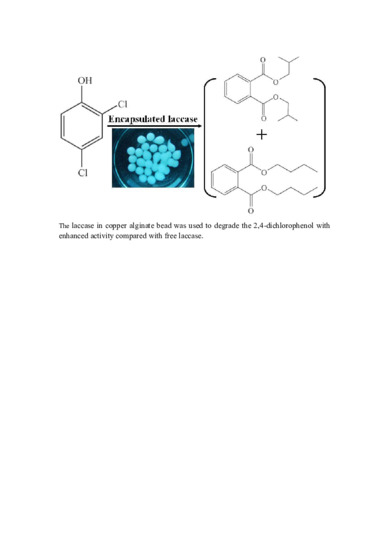
2.6. Degradation of 2,4-Dichlorophenol
2.7. Reusability
3. Materials and Methods
3.1. Materials
3.2. Purification of Laccase
3.3. Entrapment of Laccase with Different Protocols
3.4. Calculation of Specific Activity, Immobilization Yield
3.4.1. Immobilization Yield
3.4.2. Specific Activity
3.5. FTIR
3.6. The Effect of Alginate Concentration
3.7. pH Stability
3.8. Thermal Stability
3.9. Degradation of 2,4-Dichlorophenol
3.10. Reusability
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Tarasi, R.; Alipour, M.; Gorgannezhad, L.; Imanparast, S.; Yousefi-Ahmadipour, A.; Ramezani, A.; Ganjali, M.R.; Shafiee, A.; Faramarzi, M.A.; Khoobi, M. Laccase immobilization onto magnetic β-cyclodextrin-modified chitosan: Improved enzyme stability and efficient performance for phenolic compounds elimination. Macromol. Res. 2018, 26, 1–8. [Google Scholar] [CrossRef]
- Cusola, O.; Valls, C.; Vidal, T.; Roncero, M.B. Using electrochemical methods to study the kinetics of laccase-catalyzed oxidation of phenols. Ind. Eng. Chem. Res. 2018, 57, 2434–2439. [Google Scholar] [CrossRef]
- Yesilada, O.; Birhanli, E.; Geckil, H. Bioremediation and decolorization of textile dyes by white rot fungi and laccase enzymes. In Mycoremediation and Environmental Sustainability; Springer: Cham, Switzerland, 2018; Volume 7, pp. 121–153. [Google Scholar]
- Agrawal, P.K.; Shrivastava, R.; Verma, J. Bioremediation approaches for degradation and detoxification of polycyclic aromatic hydrocarbons. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2019; Volume 6, pp. 99–119. [Google Scholar]
- Rocha-Martín, J.; Martínez-Bernal, C.; Zamorano, L.S.; Reyes-Sosa, F.M.; Díez García, B. Inhibition of enzymatic hydrolysis of pretreated corn stover and sugar cane straw by laccases. Process Biochem. 2018, 67, 88–91. [Google Scholar] [CrossRef]
- Xu, F. Oxidation of phenols, anilines, and benzenethiols by fungal laccases: Correlation between activity and redox potentials as well as halide inhibition. Biochemistry 1996, 35, 7608–7614. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.S.; Kunamneni, A. Laccases—properties and applications. In Enzymes in Human and Animal Nutrition; Elsevier: Chennai, India, 2018; Volume 7, pp. 133–161. [Google Scholar]
- Brugnari, T.; Pereira, M.G.; Bubna, G.A.; de Freitas, E.N.; Contato, A.G.; Corrêa, R.C.G.; Castoldi, R.; de Souza, C.G.M.; de Moraes, M.d.L.T.; Bracht, A. A highly reusable MANAE-agarose-immobilized Pleurotus ostreatus laccase for degradation of bisphenol A. Sci. Total Environ. 2018, 634, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Khorshidi, A.; Shojaei, A.F.; Rezaei, S.; Faramarzi, M.A. Immobilization of laccase on modified Fe3O4@SiO2@kit-6 magnetite nanoparticles for enhanced delignification of olive pomace bio-waste. Int. J. Biol. Macromol. 2018, 114, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Míguez, N.; Gimeno-Pérez, M.; Fernández-Polo, D.; Cervantes, F.; Ballesteros, A.; Fernández-Lobato, M.; Ribeiro, M.; Plou, F. Immobilization of the β-fructofuranosidase from Xanthophyllomyces Dendrorhous by entrapment in polyvinyl alcohol and its application to neo-fructooligosaccharides production. Catalysts 2018, 8, 201–212. [Google Scholar] [CrossRef]
- Petrovičová, T.; Markošová, K.; Hegyi, Z.; Smonou, I.; Rosenberg, M.; Rebroš, M. Co-immobilization of ketoreductase and glucose dehydrogenase. Catalysts 2018, 8, 168–176. [Google Scholar] [CrossRef]
- Facchini, F.; Pereira, M.; Vici, A.; Filice, M.; Pessela, B.; Guisan, J.; Fernandez-Lorente, G.; Polizeli, M. Immobilization effects on the catalytic properties of two Fusarium Verticillioides lipases: Stability, hydrolysis, transesterification and enantioselectivity improvement. Catalysts 2018, 8, 84–100. [Google Scholar] [CrossRef]
- Rogalski, J.; Dawidowicz, A.; Jóźwik, E.; Leonowicz, A. Immobilization of laccase from cerrena unicolor on controlled porosity glass. J. Mol. Catal. B: Enzym. 1999, 6, 29–39. [Google Scholar] [CrossRef]
- Dodor, D.E.; Hwang, H.-M.; Ekunwe, S.I. Oxidation of anthracene and benzo[a]pyrene by immobilized laccase from trametes versicolor. Enzym. Microb. Technol. 2004, 35, 210–217. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, X.; Hwang, H.-M. Comparative study of immobilized Trametes versicolor laccase on nanoparticles and kaolinite. Chemosphere 2007, 66, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Huber, D.; Pellis, A.; Daxbacher, A.; Nyanhongo, G.S.; Guebitz, G.M. Polymerization of various lignins via immobilized myceliophthora thermophila laccase (mtl). Polymers 2016, 8, 280–289. [Google Scholar] [CrossRef]
- Huber, D.; Bleymaier, K.; Pellis, A.; Vielnascher, R.; Daxbacher, A.; Greimel, K.J.; Guebitz, G.M. Laccase catalyzed elimination of morphine from aqueous systems. New Biotechnol. 2018, 42, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Daâssi, D.; Rodríguez-Couto, S.; Nasri, M.; Mechichi, T. Biodegradation of textile dyes by immobilized laccase from Coriolopsis Gallica into Ca-alginate beads. Int. Biodeterior. Biodegrad. 2014, 90, 71–78. [Google Scholar] [CrossRef]
- Shojaat, R.; Saadatjoo, N.; Karimi, A.; Aber, S. Simultaneous adsorption–degradation of organic dyes using MnFe2O4/calcium alginate nano-composites coupled with gox and laccase. J. Environ. Chem. Eng. 2016, 4, 1722–1730. [Google Scholar] [CrossRef]
- Ouwerx, C.; Velings, N.; Mestdagh, M.; Axelos, M. Physico-chemical properties and rheology of alginate gel beads formed with various divalent cations. Polym. Gels Netw. 1998, 6, 393–408. [Google Scholar] [CrossRef]
- Singh, L.; Pavankumar, A.R.; Lakshmanan, R.; Rajarao, G.K. Effective removal of cu2+ ions from aqueous medium using alginate as biosorbent. Ecol. Eng. 2012, 38, 119–124. [Google Scholar] [CrossRef]
- Phetsom, J.; Khammuang, S.; Suwannawong, P.; Sarnthima, R. Copper-alginate encapsulation of crude laccase from Lentinus Polychrous Lev. and their effectiveness in synthetic dyes decolorizations. J. Biol. Sci. 2009, 9, 573–583. [Google Scholar]
- Teerapatsakul, C.; Bucke, C.; Parra, R.; Keshavarz, T.; Chitradon, L. Dye decolorisation by laccase entrapped in copper alginate. World J. Microbiol. Biotechnol. 2008, 24, 1367–1374. [Google Scholar] [CrossRef]
- Cao, L. Unconventional enzyme immobilization. In Carrier-Bound Immobilized Enzymes; Wiley-VCH Verlag GmbH & Co. KGaA: Delft, The Netherlands, 2006; Volume 6, pp. 449–549. [Google Scholar]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. The influence of macromolecular crowding and macromolecular confinement on biochemical reactions in physiological media. J. Biol. Chem. 2001, 276, 10577–10580. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-X.; Dill, K.A. Stabilization of proteins in confined spaces. Biochemistry 2001, 40, 11289–11293. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-B.; Wang, Y.-C.; He, R.; Zhuang, A.; Wang, X.; Zeng, J.; Hou, J. A new nanobiocatalytic system based on allosteric effect with dramatically enhanced enzymatic performance. J. Am. Chem. Soc. 2013, 135, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lei, J.; Zare, R.N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal–carboxylate interactions in metal–alginate complexes studied with FTIR spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-J.; Griffiths, P.R.; Byler, D.M.; Susi, H. Protein conformation by infrared spectroscopy: Resolution enhancement by fourier self-deconvolution. Appl. Spectrosc. 1985, 39, 282–287. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [PubMed]
- Klein, J.; Stock, J.; Vorlop, K.D. Pore size and properties of spherical Ca-alginate biocatalysts. European J. Appl. Microbiol. Biotechnol. 1983, 18, 86–91. [Google Scholar] [CrossRef]
- Velings, N.M.; Mestdagh, M.M. Physico-chemical properties of alginate gel beads. Polym. Gels Netw. 1995, 3, 311–330. [Google Scholar] [CrossRef]
- Lee, C.-H.; Lin, T.-S.; Mou, C.-Y. Mesoporous materials for encapsulating enzymes. Nano Today 2009, 4, 165–179. [Google Scholar] [CrossRef]
- Berrio, J.; Plou, F.J.; Ballesteros, A.; Martínez, Á.T.; Martínez, M.J. Immobilization of Pycnoporus coccineus laccase on Eupergit C: Stabilization and treatment of olive oil mill wastewaters. Biocatal. Biotransform. 2007, 25, 130–134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Gao, E.; Xia, L. Dechlorination of dichlorophenol in waste water by immobilized laccase. J. Chem. Soc. Perkin Trans. 2 2006, 57, 359–362. [Google Scholar]
- Sanlıer, S.H.; Gider, S.; Köprülü, A. Immobilization of laccase for biotechnology applications. Artif. Cell Nano. Bio. 2013, 41, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Seckler, R.; Jaenicke, R. Protein folding and protein refolding. FASEB J. 1992, 6, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.N.; Balkus Jr, K.J. Perspective of recent progress in immobilization of enzymes. ACS Catal. 2011, 1, 956–968. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of one-electron transfer reactions: Pulse radiolysis studies of 2, 2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 2 1982, 0, 805–812. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Y.; Yang, Y.; Yuan, H.; Liu, X. Cagelike mesoporous silica encapsulated with microcapsules for immobilized laccase and 2, 4-DCP degradation. J. Environ. Sci. 2015, 38, 52–62. [Google Scholar] [CrossRef] [PubMed]






| Samples | Specific Activity (μmol/mg/min) |
|---|---|
| Protocol I | 659.1 ± 16.4 |
| Protocol II | 119.4 ± 18.3 |
| Protocol III | 88.9 ± 15.5 |
| Protocol IV | 82.5 ± 12 .9 |
| Free laccase | 217.0 ± 11.2 |
| Free laccase [a] | 499.1 ± 17.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Wu, Z.; Chen, G.; Wang, Z. An Improved Method to Encapsulate Laccase from Trametes versicolor with Enhanced Stability and Catalytic Activity. Catalysts 2018, 8, 286. https://doi.org/10.3390/catal8070286
Zhang S, Wu Z, Chen G, Wang Z. An Improved Method to Encapsulate Laccase from Trametes versicolor with Enhanced Stability and Catalytic Activity. Catalysts. 2018; 8(7):286. https://doi.org/10.3390/catal8070286
Chicago/Turabian StyleZhang, Sitong, Zhuofu Wu, Guang Chen, and Zhi Wang. 2018. "An Improved Method to Encapsulate Laccase from Trametes versicolor with Enhanced Stability and Catalytic Activity" Catalysts 8, no. 7: 286. https://doi.org/10.3390/catal8070286
APA StyleZhang, S., Wu, Z., Chen, G., & Wang, Z. (2018). An Improved Method to Encapsulate Laccase from Trametes versicolor with Enhanced Stability and Catalytic Activity. Catalysts, 8(7), 286. https://doi.org/10.3390/catal8070286