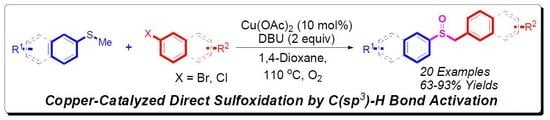
Direct Sulfoxidation of Aromatic Methyl Thioethers with Aryl Halides by Copper-Catalyzed C(sp3)–H Bond Activation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. General Synthesis Methods of 3a–5f
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef] [PubMed]
- Lenstra, D.C.; Vedovato, V.; Ferrer Flegeau, E.; Maydom, J.; Willis, M.C. One-pot sulfoxide synthesis exploiting a sulfinyl-dication equivalent generated from a DABSO/trimethylsilyl chloride sequence. Org. Lett. 2016, 18, 2086–2089. [Google Scholar] [CrossRef] [PubMed]
- Surmiak, S.K.; Doerenkamp, C.; Selter, P.; Peterlechner, M.; Schäfer, A.H.; Eckert, H.; Studer, A. Palladium Nanoparticle Loaded Bifunctional Silica Hybrid Material: Preparation and Applications as Catalyst in Hydrogenation Reactions. Chem.-A Eur. J. 2017, 23, 6019–6028. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Wang, B.; Li, B. The regioselective synthesis of 2-phosphinoylindoles via Rh (III)-catalyzed C–H activation. Org. Chem. Front. 2018, 5, 88–91. [Google Scholar] [CrossRef]
- Mishra, S.K.; Tripathi, G.; Kishore, N.; Singh, R.K.; Singh, A.; Tiwari, V.K. Drug development against tuberculosis: Impact of alkaloids. Eur. J. Med. Chem. 2017, 504–544. [Google Scholar] [CrossRef] [PubMed]
- Ntte, K.; Li, W.; Zhou, S.; Neumann, H.; Wu, X.F. Iron-catalyzed reduction of aromatic aldehydes with paraformaldehyde and H2O as the hydrogen source. Tetrahedron Lett. 2015, 56, 1118–1121. [Google Scholar] [CrossRef]
- Mukherjee, M.M.; Basu, N.; Ghosh, R. Iron (III) chloride modulated selective 1, 2-trans glycosylation based on glycosyl trichloroacetimidate donors and its application in orthogonal glycosylation. RSC Adv. 2016, 6, 105589–105606. [Google Scholar] [CrossRef]
- Weix, D.J. Methods and Mechanisms for Cross-Electrophile Coupling of Csp2 Halides with Alkyl Electrophiles. Accounts Chem. Res. 2015, 48, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Luo, K.; Yu, X.; Yang, W.-C.; Wu, L.; Zhang, W.-H. Tert-Butyl Nitrite Mediated Expeditious Methylsulfoxidation of Tetrazole-amines with DMSO: Metal-free Synthesis of Antifungal Active Methylsulfinyl-1H-tetrazole Derivatives. Adv. Synth. Catal. 2018, 360, 468–473. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-Catalyzed Ligand-Directed C−H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Shan, G.; Wang, L.; Rao, Y. Recent advances in transition metal (Pd, Ni)-catalyzed C(sp3)–H bond activation with bidentate directing groups. Tetrahedron Lett. 2016, 57, 819–836. [Google Scholar] [CrossRef]
- He, J.; Li, S.; Deng, Y. Ligand-Controlled C(sp3)–H Arylation and Olefination in Synthesis of Unnatural Chiral α–Amino Acids. Science 2014, 343, 1216–1220. [Google Scholar] [CrossRef] [PubMed]
- Duan, F.F.; Song, S.Q.; Xu, R.S. Iron(II)-catalyzed sulfur directed C(sp3)–H bond amination/C–S cross coupling reaction. Chem. Commun. 2017, 53, 2737–2739. [Google Scholar] [CrossRef] [PubMed]
- Scheer, A.M.; Eskola, A.J.; Osborn, D.L.; Sheps, L.; Taatjes, C.A. Resonance Stabilization Effects on Ketone Autoxidation: Isomer-Specific Cyclic Ether and Ketohydroperoxide Formation in the Low-Temperature (400–625 K) Oxidation of Diethyl Ketone. J. Phys. Chem. A 2016, 120, 8625–8636. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.S.; Shakhmin, A.; Glinton, K.E.; Rao, S.; Mathew, T.; Olah, G.A. Poly(N-vinylpyrrolidone)–H2O2 and poly(4-vinylpyridine)–H2O2 complexes: Solid H2O2 equivalents for selective oxidation of sulfides to sulfoxides and ketones to gem-dihydroperoxides. Green Chem. 2014, 16, 3616–3622. [Google Scholar] [CrossRef]
- Song, J.; Liu, Q.; Liu, H.; Jiang, X. Recent Advances in Palladium-Catalyzed Carboxylation with CO2. Eur. J. Org. Chem. 2018, 6, 696–713. [Google Scholar] [CrossRef]
- Caglioti, L.; Micskei, K.; Tacconi, L.; Zucchi, C.; Palyi, G. Carbon dioxide: A C-1 source for chemical industry. Chem. Today 2009, 27, 18–22. [Google Scholar]





 | ||||
|---|---|---|---|---|
| Entry | Catalyst | Base | 1a:2a | 3a [%] b |
| 1 | CuBr | DBU | 1:1 | nr |
| 2 | CuCO3 | DBU | 1:1 | 37 |
| 3 | CuSO4 | DBU | 1:1 | 45 |
| 4 | CuCl2 | DBU | 1:1 | 40 |
| 5 | CuBr2 | DBU | 1:1 | 56 |
| 6 | Cu(OAc)2 | DBU | 1:1 | 78 |
| 7 | Cu(OAc)2 | Imidazole | 1:1.2 | 31 |
| 8 | Cu(OAc)2 | Piperidine | 1:1.2 | 66 |
| 9 | Cu(OAc)2 | N,N-dimethylaniline | 1:1.2 | 51 |
| 10 | Cu(OAc)2 | tri-n-propylamine | 1:1.2 | nr |
| 11 | Cu(OAc)2 | DABCO h | 1:1.2 | 55 |
| 12 | Cu(OAc)2 | DBU | 1:1.2 | 87 |
| 13 | Cu(OAc)2 | DBU | 1:1.2 | 69 c |
| 14 | Cu(OAc)2 | DBU | 1:1.2 | 76 d |
| 15 | Cu(OAc)2 | DBU | 1:1.2 | 74 e |
| 16 | Cu(OAc)2 | DBU | 1:1.2 | 5 f |
| 17 | Cu(OAc)2 | DBU | 1:1.2 | 0 g |
 | ||||
|---|---|---|---|---|
| Entry | 1 | 2 | 3 | Yield b (Yield c) |
| 1 |  |  |  | 87 (83) |
| 2 |  |  |  | 83 (80) |
| 3 |  |  |  | 84 (81) |
| 4 |  |  |  | 93 (92) |
| 5 |  |  |  | 88 (87) |
| 6 |  |  |  | 90 (81) |
| 7 |  |  |  | 87 (83) |
| 8 |  |  |  | 81 (80) |
| 9 |  |  |  | 85 (79) |
| 10 |  |  |  | 80(77) |
| 11 |  |  |  | 75 (70) |
| 12 |  |  |  | 79 (74) |
| 13 |  |  |  | 74 (69) |
| 14 |  |  |  | 72 (65) |
 | ||||
|---|---|---|---|---|
| Entry | 1 | 4 | 5 | Yield b (Yield c) |
| 1 |  |  |  | 79 (70) |
| 2 |  |  |  | 87 (79) |
| 3 |  |  |  | 63 (54) |
| 4 |  |  |  | 90 (82) |
| 5 |  |  |  | 81(76) |
| 6 |  |  |  | 86 (78) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Zhu, Y.; Xiong, F.; Tong, S. Direct Sulfoxidation of Aromatic Methyl Thioethers with Aryl Halides by Copper-Catalyzed C(sp3)–H Bond Activation. Catalysts 2019, 9, 105. https://doi.org/10.3390/catal9010105
Xu R, Zhu Y, Xiong F, Tong S. Direct Sulfoxidation of Aromatic Methyl Thioethers with Aryl Halides by Copper-Catalyzed C(sp3)–H Bond Activation. Catalysts. 2019; 9(1):105. https://doi.org/10.3390/catal9010105
Chicago/Turabian StyleXu, Runsheng, Yueer Zhu, Feixiang Xiong, and Suli Tong. 2019. "Direct Sulfoxidation of Aromatic Methyl Thioethers with Aryl Halides by Copper-Catalyzed C(sp3)–H Bond Activation" Catalysts 9, no. 1: 105. https://doi.org/10.3390/catal9010105
APA StyleXu, R., Zhu, Y., Xiong, F., & Tong, S. (2019). Direct Sulfoxidation of Aromatic Methyl Thioethers with Aryl Halides by Copper-Catalyzed C(sp3)–H Bond Activation. Catalysts, 9(1), 105. https://doi.org/10.3390/catal9010105