The Selective Oxidation of Sulfides to Sulfoxides or Sulfones with Hydrogen Peroxide Catalyzed by a Dendritic Phosphomolybdate Hybrid
Abstract
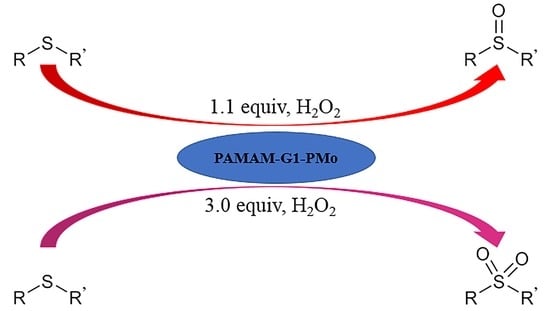
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Preparation and Characterization of PAMAM-G1-PMo
3.2. Catalytic Reactions
3.2.1. General Experimental Procedure for the Oxidation of Sulfides to Sulfoxides
3.2.2. General Experimental Procedure for the Oxidation of Sulfides to Sulfones
3.2.3. Synthesis of Modafinil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sipos, G.; Drinkel, E.E.; Dorta, R. The emergence of sulfoxides as efficient ligands in transition metal catalysis. Chem. Soc. Rev. 2015, 44, 3834–3860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Liu, Y.; Li, Y.; Wang, Q. Design, Synthesis, Acaricidal/Insecticidal Activity, and Structure-Activity Relationship Studies of Novel Oxazolines Containing Sulfone/Sulfoxide Groups Based on the Sulfonylurea Receptor Protein-Binding Site. J. Agric. Food Chem. 2016, 64, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Skoda, E.M.; Sacher, J.R.; Kazancioglu, M.Z.; Saha, J.; Wipf, P. An uncharged oxetanyl sulfoxide as a covalent modifier for improving aqueous solubility. ACS Med. Chem. Lett. 2014, 5, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Petroff, J.T.; Skubic, K.N.; Arnatt, C.K.; McCulla, R.D. Asymmetric Dibenzothiophene Sulfones as Fluorescent Nuclear Stains. J. Org. Chem. 2018, 83, 14063–14068. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Xiao, G.; Wang, Y.; Zi, G.; Zhang, Z.; Hou, G. Highly Efficient Enantioselective Synthesis of Chiral Sulfones by Rh-Catalyzed Asymmetric Hydrogenation. J. Am. Chem. Soc. 2019, 141, 1749–1756. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, R.D.; Ramkumar, V.; Chand, D.K. Molybdenum based metallomicellar catalyst for controlled and selective sulfoxidation reactions in aqueous medium. Green Chem. 2014, 16, 2190–2196. [Google Scholar] [CrossRef]
- Clifford, G.V.; Squires, T.G.; Chen, Y.Y. Peroxytrifluoroacetic Acid. A Convenient Reagent for the Preparation of Sulfoxides and Sulfones. J. Org. Chem. 1982, 47, 3773–3774. [Google Scholar]
- Kowalski, P.; Mitka, K.; Ossowska, K.; Kolarska, Z. Oxidation of sulfides to sulfoxides. Part 1: Oxidation using halogen derivatives. Tetrahedron 2005, 61, 1933–1953. [Google Scholar] [CrossRef]
- Yang, C.; Jin, Q.; Zhang, H.; Liao, J.; Zhu, J.; Yu, B.; Deng, J. Tetra-(tetraalkylammonium)octamolybdate catalysts for selective oxidation of sulfides to sulfoxides with hydrogen peroxide. Green Chem. 2009, 11, 1401–1405. [Google Scholar] [CrossRef]
- Zhang, M.; Pan, Y.; Dorfman, R.G.; Yin, Y.; Zhou, Q.; Huang, S.; Liu, J.; Zhao, S. Sirtinol promotes PEPCK1 degradation and inhibits gluconeogenesis by inhibiting deacetylase SIRT2. Sci. Rep. 2017, 7, 7. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, C.X.; Cheng, Z.G.; Zhang, Z.H. A reusable catalytic system for sulfide oxidation and epoxidation of allylic alcohols in water catalyzed by poly(dimethyl diallyl) ammonium/polyoxometalate. Green Chem. 2016, 18, 995–998. [Google Scholar] [CrossRef]
- Mary Imelda Jayaseeli, A.; Ramdass, A.; Rajagopal, S. Selective H2O2 oxidation of organic sulfides to sulfoxides catalyzed by cobalt(III)–salen ion. Polyhedron 2015, 100, 59–66. [Google Scholar] [CrossRef]
- Jafarpour, M.; Rezaeifard, A.; Ghahramaninezhad, M.; Feizpour, F. Dioxomolybdenum(vi) complex immobilized on ascorbic acid coated TiO2 nanoparticles catalyzed heterogeneous oxidation of olefins and sulfides. Green Chem. 2015, 17, 442–452. [Google Scholar] [CrossRef]
- Gogoi, P.; Kalita, M.; Bhattacharjee, T.; Barman, P. Copper–Schiff base complex catalyzed oxidation of sulfides with hydrogen peroxide. Tetrahedron Lett. 2014, 55, 1028–1030. [Google Scholar] [CrossRef]
- Jain, S.L.; Rana, B.S.; Singh, B.; Sinha, A.K.; Bhaumik, A.; Nandi, M.; Sain, B. An improved high yielding immobilization of vanadium Schiff base complexes on mesoporous silica via azide–alkyne cycloaddition for the oxidation of sulfides. Green Chem. 2010, 12, 374–377. [Google Scholar] [CrossRef]
- Jiang, J.; Luo, R.C.; Zhou, X.T.; Chen, Y.J.; Jia, H.B. Photocatalytic Properties and Mechanistic Insights into Visible Light-Promoted Aerobic Oxidation of Sulfides to Sulfoxides via Tin Porphyrin-Based Porous Aromatic Frameworks. Adv. Synth. Catal. 2018, 360, 4402–4411. [Google Scholar] [CrossRef]
- Heidari, L.; Shiri, L. CoFe2O4@SiO2-CPTES-Guanidine-Cu(II): A novel and reusable nanocatalyst for the synthesis of 2,3-dihydroquinazolin-4(1H)-ones and polyhydroquinolines and oxidation of sulfides. Appl. Organomet Chem. 2019, 33, e4636. [Google Scholar] [CrossRef]
- Han, M.; Niu, Y.; Wan, R.; Xu, Q.; Lu, J.; Ma, P.; Zhang, C.; Niu, J.; Wang, J. A Crown-Shaped Ru-Substituted Arsenotungstate for Selective Oxidation of Sulfides with Hydrogen Peroxide. Chem. Eur. J. 2018, 24, 11059–11066. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Hyodo, M.; Aoki, M.; Zheng, X.Q.; Noyori, R. Oxidation of sulfides to sulfoxides and sulfones with 30% hydrogen peroxide under organic solvent- and halogen-free conditions. Tetrahedron 2001, 57, 2469–2476. [Google Scholar] [CrossRef]
- Yvon, C.; Surman, A.J.; Hutin, M.; Alex, J.; Smith, B.O.; Long, D.L.; Cronin, L. Polyoxometalate clusters integrated into peptide chains and as inorganic amino acids: Solution- and solid-phase approaches. Angew. Chem. Int. Ed. 2014, 53, 3336–3341. [Google Scholar] [CrossRef]
- Fu, H.; Qin, C.; Lu, Y.; Zhang, Z.M.; Li, Y.G.; Su, Z.M.; Li, W.L.; Wang, E.B. An ionothermal synthetic approach to porous polyoxometalate-based metal-organic frameworks. Angew. Chem. Int. Ed. 2012, 51, 7985–7989. [Google Scholar] [CrossRef] [PubMed]
- Nohra, B.; El Moll, H.; Rodriguez Albelo, L.M.; Mialane, P.; Marrot, J.; Mellot-Draznieks, C.; O’Keeffe, M.; Ngo Biboum, R.; Lemaire, J.; Keita, B.; et al. Polyoxometalate-based metal organic frameworks (POMOFs): Structural trends, energetics, and high electrocatalytic efficiency for hydrogen evolution reaction. J. Am. Chem. Soc. 2011, 133, 13363–13374. [Google Scholar] [CrossRef] [PubMed]
- Long, D.L.; Burkholder, E.; Cronin, L. Polyoxometalate clusters, nanostructures and materials: From self assembly to designer materials and devices. Chem. Soc. Rev. 2007, 36, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.J.; Liu, S.X.; Sun, C.Y.; Liang, D.D.; Ren, G.J.; Wei, F.; Chen, Y.G.; Su, Z.M. A sodalite-type porous metal-organic framework with polyoxometalate templates: Adsorption and decomposition of dimethyl methylphosphonate. J. Am. Chem. Soc. 2011, 133, 4178–4181. [Google Scholar] [CrossRef] [PubMed]
- Poli, E.; De Sousa, R.; Jérome, F.; Pouilloux, Y.; Clacens, J.M. Catalytic epoxidation of styrene and methyl oleate over peroxophosphotungstate entrapped in mesoporous SBA-15. Catal. Sci. Technol. 2012, 2, 910–914. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhang, J.; Zou, X.; Dong, H.; Yao, P. Selective oxidation of styrene to 1,2-epoxyethylbenzene by hydrogen peroxide over heterogeneous phosphomolybdic acid supported on ionic liquid modified MCM-41. Chem. Eng. J. 2015, 260, 172–177. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Zhou, F.; Wang, Y.; Yuan, X.; Wang, H. Oxidative desulfurization of dibenzothiophene and diesel by hydrogen peroxide: Catalysis of H3PMo12O40 immobilized on the ionic liquid modified SiO2. Mol. Catal. 2018, 452, 93–99. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, Y.; Ma, B.; Ding, Y.; Qiu, W. Oxidation of alcohols with hydrogen peroxide in water catalyzed by recyclable keggin-type tungstoborate catalyst. Catal. Commun. 2010, 11, 527–531. [Google Scholar] [CrossRef]
- Ichihara, J.; Yamaguchi, S.; Nomoto, T.; Nakayama, H.; Iteya, K.; Naitoh, N.; Sasakic, Y. Keggin-type polyacid clusters on apatite: Characteristic catalytic activities in solvent-free oxidation. Tetrahedron Lett. 2002, 43, 8231–8234. [Google Scholar] [CrossRef]
- Méry, D.; Astruc, D. Dendritic catalysis: Major concepts and recent progress. Coord. Chem. Rev. 2006, 250, 1965–1979. [Google Scholar] [CrossRef]
- Peng, M.; Chen, Y.; Tan, R.; Zheng, W.; Yin, D. A highly efficient and recyclable catalyst—Dendrimer supported chiral salen Mn(iii) complexes for asymmetric epoxidation. RSC Adv. 2013, 3, 20684–20692. [Google Scholar] [CrossRef]
- Caminade, A.M.; Ouali, A.; Keller, M.; Majoral, J.P. Organocatalysis with dendrimers. Chem. Soc. Rev. 2012, 41, 4113–4125. [Google Scholar] [CrossRef] [PubMed]
- Breinbauer, R.; Jacobsen, E.N. Cooperative Asymmetric Catalysis with Dendrimeric [Co(salen)] Complexes. Angew. Chem. Int. Ed. 2000, 39, 3604–3607. [Google Scholar] [CrossRef]
- Nlate, S.; Jahier, C. Dendritic Polyoxometalate Hybrids: Efficient and Recoverable Catalysts for Oxidation Reactions. Eur. J. Inorg. Chem. 2013, 2013, 1606–1619. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, R.; Zheng, W.; Zhang, Y.; Zhao, G.; Yin, D. Dendritic phosphotungstate hybrids efficiently catalyze the selective oxidation of alcohols with H2O2. Catal. Sci. Technol. 2014, 4, 4084–4092. [Google Scholar] [CrossRef]
- Fornaroli, M.; Velardi, F.; Colli, C.; Baima, R. Process for the synthesis of Modafinil. U.S. Patent US 2004/0106829 A1, 3 June 2004. [Google Scholar]






| Entry | Catalyst | Time (h) | Temperature (°C) | Yield b (%) |
|---|---|---|---|---|
| 1 | — | 6.0 | 25 | 4 |
| 2 | HPMo | 8.0 | 25 | 8 |
| 3 | PAMAM-G1 | 8.0 | 25 | 0.5 |
| 4 | PAMAM-G1-PMo | 4.0 | 25 | 85 |

| Entry | Solvent | Time (h) | Temperature (°C) | Yield b (%) |
|---|---|---|---|---|
| 1 | MeOH | 4.0 | 25 | 90 |
| 2 | 95%EtOH | 4.0 | 25 | 85 |
| 3 | EtOH | 4.0 | 25 | 60 |
| 4 | CH3COCH3 | 4.0 | 25 | 45 |
| 5 | CH3COOC2H5 | 4.0 | 25 | 40 |
| 6 | i-PrOH | 4.0 | 25 | 52 |
| 7 | 95%EtOH | 2.0 | 25 | 72 |
| 8 | 95%EtOH | 2.0 | 30 | 91 |
| 9 | 95%EtOH | 2.0 | 35 | 88 |

| Entry | 30 wt% H2O2 (mol%) | Time (h) | Temperature (°C) | Yield b (%) |
|---|---|---|---|---|
| 1 | 300 | 3.0 | 30 | 60 |
| 2 | 300 | 3.0 | 40 | 93 |
| 3 | 300 | 3.0 | 50 | 92 |
| 4 | 400 | 3.0 | 40 | 93 |
| 5 | 200 | 3.0 | 40 | 81 |
| Entry | Substrates | Products | Time (h) | Yield c (%) |
|---|---|---|---|---|
| 1 |  1a 1a |  1b 1b | 2.0 | 91 |
| 2 |  2a 2a |  2b 2b | 1.5 | 90 |
| 3 |  3a 3a |  3b 3b | 3.0 | 85 |
| 4 |  4a 4a |  4b 4b | 3.5 | 85 |
| 5 |  5a 5a |  5b 5b | 5.0 | trace |
| 6 |  6a 6a |  6b 6b | 6.0 | 93 |
| 7 |  7a 7a |  7b 7b | 4.5 | 93 |
| 8 |  1a 1a |  1c 1c | 3.5 | 93 |
| 9 |  2a 2a |  2c 2c | 2.0 | 94 |
| 10 |  3a 3a |  3c 3c | 4.0 | 92 |
| 11 |  4a 4a |  4c 4c | 4.0 | 93 |
| 12 |  5a 5a |  5c 5c | 5.0 | NA d |
| 13 |  6a 6a |  6c 6c | 5.0 | trace |
| 14 |  7a 7a |  7c 7c | 4.0 | 91 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Q.-L.; Fan, Z.-F.; Yang, J.-W.; Li, Q.; Chen, Y.-X.; Cheng, M.-S.; Liu, Y. The Selective Oxidation of Sulfides to Sulfoxides or Sulfones with Hydrogen Peroxide Catalyzed by a Dendritic Phosphomolybdate Hybrid. Catalysts 2019, 9, 791. https://doi.org/10.3390/catal9100791
Tong Q-L, Fan Z-F, Yang J-W, Li Q, Chen Y-X, Cheng M-S, Liu Y. The Selective Oxidation of Sulfides to Sulfoxides or Sulfones with Hydrogen Peroxide Catalyzed by a Dendritic Phosphomolybdate Hybrid. Catalysts. 2019; 9(10):791. https://doi.org/10.3390/catal9100791
Chicago/Turabian StyleTong, Qiao-Lin, Zhan-Fang Fan, Jian-Wen Yang, Qi Li, Yi-Xuan Chen, Mao-Sheng Cheng, and Yang Liu. 2019. "The Selective Oxidation of Sulfides to Sulfoxides or Sulfones with Hydrogen Peroxide Catalyzed by a Dendritic Phosphomolybdate Hybrid" Catalysts 9, no. 10: 791. https://doi.org/10.3390/catal9100791
APA StyleTong, Q. -L., Fan, Z. -F., Yang, J. -W., Li, Q., Chen, Y. -X., Cheng, M. -S., & Liu, Y. (2019). The Selective Oxidation of Sulfides to Sulfoxides or Sulfones with Hydrogen Peroxide Catalyzed by a Dendritic Phosphomolybdate Hybrid. Catalysts, 9(10), 791. https://doi.org/10.3390/catal9100791