Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane
Abstract
:1. Introduction
2. Results and Discussion
2.1. The BET Results of Y-Doped and Y-Free NiO–ZrOm Catalysts
2.2. The Reducibility of Y-Doped and Y-Free NiO–ZrOm Catalysts
2.3. Basicity of Y-Doped and Y-Free NiO–ZrOm Catalysts
2.4. Nickel Particle Size and Crystallized Phases of Y-Doped and Y-Free NiO–ZrOm Catalysts
2.5. The Performance of Y-Doped and Y-Free NiO–ZrOm Catalysts
2.6. On the Carbon Deposition on Used Y-Doped and Y-Free NiO–ZrOm Catalysts
2.6.1. Carbon Formation Evidenced by XPS and Raman Spectroscopy
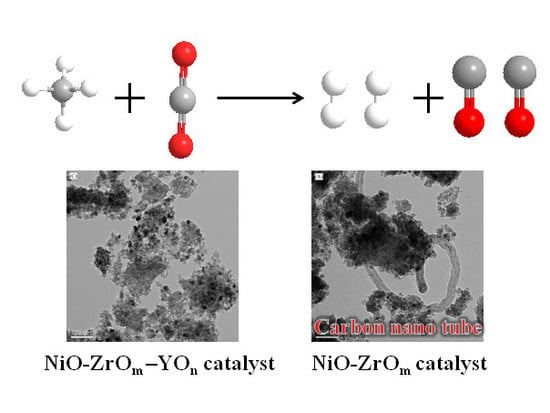
2.6.2. On the Study of the Morphology of Carbon Studied by TEM
3. Materials and Methods
3.1. Synthesis of Y Doped NiO–ZrOm and NiO–ZrOm Catalysts
3.2. Activity Test
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiao, F.; Li, J.; Pan, X.; Xiao, J.; Li, H.; Ma, H.; Wei, M.; Pan, Y.; Zhou, Z.; Li, M. Selective conversion of syngas to light olefins. Science 2016, 351, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.-U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, P.; Adegbite, S.; Zhao, H.; Lester, E.; Wu, T. Tuning dry reforming of methane for FT syntheses: A thermodynamic approach. Appl. Energy 2018, 227, 190–197. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Rodella, C.B.; Assaf, E.M. Oxidative reforming of model biogas over NiO–Y2O3–ZrO2 catalysts. Appl. Catal. B Environ. 2013, 132, 1–12. [Google Scholar] [CrossRef]
- Pantaleo, G.; La Parola, V.; Deganello, F.; Singha, R.; Bal, R.; Venezia, A. Ni/CeO2 catalysts for methane partial oxidation: Synthesis driven structural and catalytic effects. Appl. Catal. B Environ. 2016, 189, 233–241. [Google Scholar] [CrossRef]
- Abdullah, B.; Ghani, N.A.A.; Vo, D.-V.N. Recent advances in dry reforming of methane over Ni-based catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef] [Green Version]
- Singha, R.K.; Yadav, A.; Shukla, A.; Kumar, M.; Bal, R. Low temperature dry reforming of methane over Pd-CeO2 nanocatalyst. Catal. Commun. 2017, 92, 19–22. [Google Scholar] [CrossRef]
- Liang, Z.; Li, T.; Kim, M.; Asthagiri, A.; Weaver, J.F. Low-temperature activation of methane on the IrO2(110) surface. Science 2017, 356, 299–303. [Google Scholar] [CrossRef]
- Polo-Garzon, F.; Pakhare, D.; Spivey, J.J.; Bruce, D.A. Dry reforming of methane on Rh-doped pyrochlore catalysts: A steady-state isotopic transient kinetic study. ACS Catal. 2016, 6, 3826–3833. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, S.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206. [Google Scholar] [CrossRef]
- García-Diéguez, M.; Pieta, I.; Herrera, M.; Larrubia, M.; Alemany, L. Nanostructured Pt-and Ni-based catalysts for CO2-reforming of methane. J. Catal. 2010, 270, 136–145. [Google Scholar] [CrossRef]
- El Hassan, N.; Kaydouh, M.; Geagea, H.; El Zein, H.; Jabbour, K.; Casale, S.; El Zakhem, H.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A Gen. 2016, 520, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry reforming of methane over nickel catalysts supported on magnesium aluminate spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Christensen, K.O.; Chen, D.; Lødeng, R.; Holmen, A. Effect of supports and Ni crystal size on carbon formation and sintering during steam methane reforming. Appl. Catal. A Gen. 2006, 314, 9–22. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Jiang, B.; Kawi, S. Sintering resistant Ni nanoparticles exclusively confined within SiO2 nanotubes for CH4 dry reforming. Catal. Sci. Technol. 2018, 8, 3363–3371. [Google Scholar] [CrossRef]
- Kim, W.Y.; Lee, Y.H.; Park, H.; Choi, Y.H.; Lee, M.H.; Lee, J.S. Coke tolerance of Ni/Al2 O3 nanosheet catalyst for dry reforming of methane. Catal. Sci. Technol. 2016, 6, 2060–2064. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Thitsartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3–Al2O3: Unique catalytic structure for stable CO2 reforming of methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional effect of alkaline earth over Ni–La2O3 catalyst for CO2 reforming of CH4: Role of surface oxygen species on H2 production and carbon suppression. Int. J. Hydrog. Energy 2011, 36, 14435–14446. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Syngas production from dry methane reforming over yttrium-promoted nickel-KIT-6 catalysts. Int. J. Hydrog. Energy 2019, 44, 274–286. [Google Scholar] [CrossRef]
- Taherian, Z.; Yousefpour, M.; Tajally, M.; Khoshandam, B. A comparative study of ZrO2, Y2O3 and Sm2O3 promoted Ni/SBA-15 catalysts for evaluation of CO2/methane reforming performance. Int. J. Hydrog. Energy 2017, 42, 16408–16420. [Google Scholar] [CrossRef]
- Li, B.; Su, W.; Wang, X.; Wang, X. Alumina supported Ni and Co catalysts modified by Y2O3 via different impregnation strategies: Comparative analysis on structural properties and catalytic performance in methane reforming with CO2. Int. J. Hydrog. Energy 2016, 41, 14732–14746. [Google Scholar] [CrossRef]
- Li, J.F.; Xia, C.; Au, C.T.; Liu, B.S. Y2O3—promoted NiO/SBA-15 catalysts highly active for CO2/CH4 reforming. Int. J. Hydrog. Energy 2014, 39, 10927–10940. [Google Scholar] [CrossRef]
- Bellido, J.D.; Assaf, E.M. Effect of the Y2O3–ZrO2 support composition on nickel catalyst evaluated in dry reforming of methane. Appl. Catal. A Gen. 2009, 352, 179–187. [Google Scholar] [CrossRef]
- Yabe, T.; Mitarai, K.; Oshima, K.; Ogo, S.; Sekine, Y. Low-temperature dry reforming of methane to produce syngas in an electric field over La-doped Ni/ZrO2 catalysts. Fuel Process. Technol. 2017, 158, 96–103. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S. Methane reforming with CO2 using nickel catalysts supported on yttria-doped SBA-15 mesoporous materials via sol–gel process. Int. J. Hydrog. Energy 2013, 38, 14250–14260. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, J.; Wu, Y.; Pan, J.; Zhang, Q.; Tan, Y.; Han, Y. Insight into the effects of the oxygen species over Ni/ZrO2 catalyst surface on methane reforming with carbon dioxide. Appl. Catal. B Environ. 2019, 244, 427–437. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, L.; Wang, Y.; Wang, S.; Zhao, Q.; Mao, D.; Hu, C. Low-temperature catalytic CO2 dry reforming of methane on Ni-Si/ZrO2 catalyst. ACS Catal. 2018, 8, 6495–6506. [Google Scholar] [CrossRef]
- Lou, Y.; Steib, M.; Zhang, Q.; Tiefenbacher, K.; Horváth, A.; Jentys, A.; Liu, Y.; Lercher, J.A. Design of stable Ni/ZrO2 catalysts for dry reforming of methane. J. Catal. 2017, 356, 147–156. [Google Scholar] [CrossRef]
- Świrk, K.; Galvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Dry reforming of methane over Zr-and Y-modified Ni/Mg/Al double-layered hydroxides. Catal. Commun. 2018, 117, 26–32. [Google Scholar] [CrossRef]
- Świrk, K.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Effect of low loading of yttrium on Ni-based layered double hydroxides in CO2 reforming of CH4. React. Kinet. Mech. Catal. 2019, 126, 611–628. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Xinmei, L.; Qian, L.; Yan, Z.-F. CO2−CH4 Reforming over Nickel Catalysts Supported on Mesoporous Nanocrystalline Zirconia with High Surface Area. Energy Fuels 2007, 21, 581–589. [Google Scholar] [CrossRef]
- Asencios, Y.J.; Nascente, P.A.; Assaf, E.M. Partial oxidation of methane on NiO–MgO–ZrO2 catalysts. Fuel 2012, 97, 630–637. [Google Scholar] [CrossRef]
- Dow, W.-P.; Wang, Y.-P.; Huang, T.-J. Yttria-stabilized zirconia supported copper oxide catalyst: I. Effect of oxygen vacancy of support on copper oxide reduction. J. Catal. 1996, 160, 155–170. [Google Scholar] [CrossRef]
- Maia, T.A.; Assaf, E.M. Catalytic features of Ni supported on CeO2–ZrO2 solid solution in the steam reforming of glycerol for syngas production. RSC Adv. 2014, 4, 31142–31154. [Google Scholar] [CrossRef]
- Imparato, C.; Fantauzzi, M.; Passiu, C.; Rea, I.; Ricca, C.; Aschauer, U.; Sannino, F.; D’Errico, G.; De Stefano, L.; Rossi, A. Unraveling the Charge State of Oxygen Vacancies in ZrO2−x on the Basis of Synergistic Computational and Experimental Evidence. J. Phys. Chem. C 2019, 123, 11581–11590. [Google Scholar] [CrossRef]
- Ingo, G.M. Origin of Darkening in 8 wt% Yttria—Zirconia Plasma-Sprayed Thermal Barrier Coatings. J. Am. Ceram. Soc. 1991, 74, 381–386. [Google Scholar] [CrossRef]
- Świrk, K.; Galvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Yttrium promoted Ni-based double-layered hydroxides for dry methane reforming. J. CO2 Util. 2018, 27, 247–258. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Influence of Ce/Zr molar ratio on catalytic performance of hydrotalcite-derived catalysts at low temperature CO2 methane reforming. Int. J. Hydrog. Energy 2017, 42, 23556–23567. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Da Costa, P.; Grzybek, T. Catalytic activity of hydrotalcite-derived catalysts in the dry reforming of methane: On the effect of Ce promotion and feed gas composition. React. Kinet. Mech. Catal. 2017, 121, 185–208. [Google Scholar] [CrossRef] [Green Version]
- Dębek, R.; Motak, M.; Duraczyska, D.; Launay, F.; Galvez, M.E.; Grzybek, T.; Da Costa, P. Methane dry reforming over hydrotalcite-derived Ni–Mg–Al mixed oxides: The influence of Ni content on catalytic activity, selectivity and stability. Catal. Sci. Technol. 2016, 6, 6705–6715. [Google Scholar] [CrossRef]
- Wang, C.; Sun, N.; Kang, M.; Wen, X.; Zhao, N.; Xiao, F.; Wei, W.; Zhao, T.; Sun, Y. The bi-functional mechanism of CH4 dry reforming over a Ni–CaO–ZrO2 catalyst: Further evidence via the identification of the active sites and kinetic studies. Catal. Sci. Technol. 2013, 3, 2435–2443. [Google Scholar] [CrossRef]
- Świrk, K.; Rønning, M.; Motak, M.; Beaunier, P.; Da Costa, P.; Grzybek, T. Ce-and Y-Modified Double-Layered Hydroxides as Catalysts for Dry Reforming of Methane: On the Effect of Yttrium Promotion. Catalysts 2019, 9, 56. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Nishida, K.; Zhan, Y.; Shishido, T.; Oumi, Y.; Sano, T.; Takehira, K. Sustainable Ru-doped Ni catalyst derived from hydrotalcite in propane reforming. Appl. Clay Sci. 2009, 43, 49–56. [Google Scholar] [CrossRef]
- Bai, T.; Zhang, X.; Wang, F.; Qu, W.; Liu, X.; Duan, C. Coking behaviors and kinetics on HZSM-5/SAPO-34 catalysts for conversion of ethanol to propylene. J. Energy Chem. 2016, 25, 545–552. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Shi, D.; Jia, X.; Wang, X.; Borgna, A.; Lau, R.; Yang, Y. Methane reforming with carbon dioxide over a Ni/ZiO2–SiO2 catalyst: Influence of pretreatment gas atmospheres. Int. J. Hydrog. Energy 2012, 37, 10135–10144. [Google Scholar] [CrossRef]






| Catalyst | DP, nm | SBET, m2/g | VP, cm3/g | H2 Consumption, mmol H2/g | Zr 3d (%) | Ni (wt %) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Theory a | α | β | Zr4+ | Zr3+ | |||||
| NiO–ZrOm–YOn | 2 | 79 | 0.1 | 0.37 | 0.39 | 0.10 | 0.27 | 16 | 84 | 10 |
| NiO–ZrOm | 2 | 113 | 0.2 | 0.62 | 0.47 | 0.13 | 0.49 | 10 | 90 | 12 |
| Catalyst | CO2 Peak Identification | ||||||
|---|---|---|---|---|---|---|---|
| Peak 1 (Weak) | Peak 2 (Medium-Strength) | Peak 3 (Strong) | Total Basicity (μmol CO2/g) | ||||
| Position (°C) | Content (%) | Position (°C) | Content (%) | Position (°C) | Content (%) | ||
| NiO–ZrOm | 185 | 15.5 | 252 | 64.4 | 380 | 20.1 | 73 |
| NiO–ZrOm–YOn | 150 | 36.9 | 240 | 55.4 | 380 | 13.0 | 100 |
| Catalyst | ZrO2 (nm) | Ni0 (nm) | Particle | Coke Content (%) | C–C Content (%) | IG/ID | ||
|---|---|---|---|---|---|---|---|---|
| Reduction | Reaction | Reduction | Reaction | Size (nm) | ||||
| NiO–ZrOm | 7 | 7 | 12 | 24 | 15–20 | 3.7 | 42 | 1.7 |
| NiO–ZrOm–YOn | 7 | 7 | 16 | 10 | 10–15 | 1.0 | 38 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, L.; Wang, Y.; Da Costa, P.; Hu, C. Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane. Catalysts 2019, 9, 1055. https://doi.org/10.3390/catal9121055
Wang Y, Li L, Wang Y, Da Costa P, Hu C. Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane. Catalysts. 2019; 9(12):1055. https://doi.org/10.3390/catal9121055
Chicago/Turabian StyleWang, Ye, Li Li, Yannan Wang, Patrick Da Costa, and Changwei Hu. 2019. "Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane" Catalysts 9, no. 12: 1055. https://doi.org/10.3390/catal9121055
APA StyleWang, Y., Li, L., Wang, Y., Da Costa, P., & Hu, C. (2019). Highly Carbon-Resistant Y Doped NiO–ZrOm Catalysts for Dry Reforming of Methane. Catalysts, 9(12), 1055. https://doi.org/10.3390/catal9121055