In-Situ Synthesis of Nb2O5/g-C3N4 Heterostructures as Highly Efficient Photocatalysts for Molecular H2 Evolution under Solar Illumination
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis Procedure
2.2. Structural and Morphological Characterization
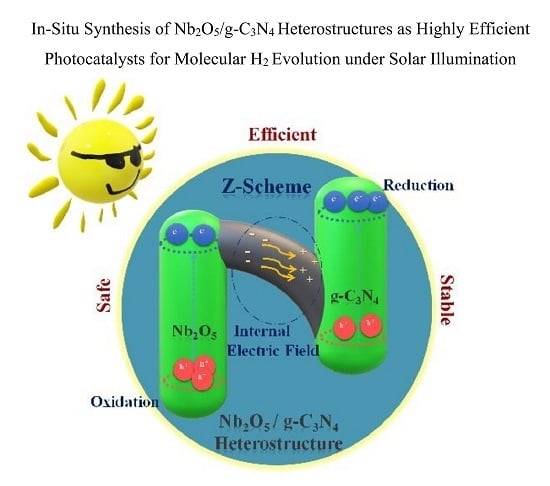
2.3. Photocatalytic Evolution of Molecular H2
3. Methods
3.1. Material Synthesis
3.1.1. Preparation of Nb2O5 (NBO)
3.1.2. Preparation of g-C3N4 (GCN)
3.1.3. Preparation of Nb2O5/g-C3N4 (NBCN)
3.2. Photodeposition of Platinum (Pt)
3.3. Material Characterization
3.4. Photocatalytic Molecular Hydrogen (H2) Formation
3.5. Photoelectrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Qi, L.; Jaronie, M. Hydrogen Production by Photocatalytic Water Splitting over Pt/TiO2 Nanosheets with Exposed (001) Facets. J. Phys. Chem. C 2010, 114, 13118–13125. [Google Scholar] [CrossRef]
- Cho, I.S.; Chen, Z.; Forman, A.J.; Kim, D.R.; Rao, P.M.; Jaramillo, T.F.; Zheng, X. Branched TiO2 Nanorods for Photoelectrochemical Hydrogen Production. Nano Lett. 2011, 11, 4978–4984. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Steier, L.; Son, M.K.; Schreier, M.; Mayer, M.T.; Michael, G. Cu2O Nanowire Photocathodes for Efficient and Durable Solar Water Splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Fang, H.; Zheng, Y.-Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef] [PubMed]
- Tahir, M.; Cao, C.; Butt, F.K.; Butt, S.; Idrees, F.; Ali, Z.; Aslam, I.; Tanveer, M.; Mahmood, A.; Mahmood, N. Large scale production of novel g-C3N4 micro strings with high surface area and versatile photodegradation ability. CrystEngComm 2014, 16, 1825–1830. [Google Scholar] [CrossRef]
- Tahir, M.; Cao, C.; Butt, F.K.; Idrees, F.; Mahmood, N.; Ali, Z.; Aslam, I.; Tanveer, M.; Rizwan, M.; Mahmood, T. Tubular graphitic-C3N4: A prospective material for energy storage and green photocatalysis. J. Mater. Chem. A 2013, 1, 13949–13955. [Google Scholar] [CrossRef]
- Yan, J.; Wu, G.; Guan, N.; Li, L. Nb2O5/TiO2 heterojunctions: synthesis strategy and photocatalytic activity. Appl. Catal. B 2014, 152, 280–288. [Google Scholar] [CrossRef]
- Tahir, M.; Cao, C.; Mahmood, N.; Butt, F.K.; Mahmood, A.; Idrees, F.; Hussain, S.; Tanveer, M.; Ali, Z.; Aslam, I. Multifunctional g-C3N4 nanofibers: A template-free fabrication and enhanced optical, electrochemical, and photocatalyst properties. ACS Appl. Mater. Interfaces 2013, 6, 1258–1265. [Google Scholar] [CrossRef]
- Sridharan, K.; Jang, E.; Park, T.J. Novel visible light active graphitic C3N4–TiO2 composite photocatalyst: Synergistic synthesis, growth and photocatalytic treatment of hazardous pollutants. Appl. Catal. B 2013, 142–143, 718–728. [Google Scholar] [CrossRef]
- Hou, Y.; Zhu, Y.; Xu, Y.; Wang, X. Photocatalytic hydrogen production over carbon nitride loaded with WS2 as cocatalyst under visible light. Appl. Catal. B 2014, 156–157, 122–127. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, P.-Q.; Liu, J.-Y.; Liu, X.-J. Enhanced photocatalytic performance of direct Z-scheme BiOCl-g-C3N4 photocatalysts. RSC Adv. 2014, 4, 19456–19461. [Google Scholar] [CrossRef]
- Aslam, I.; Cao, C.; Tanveer, M.; Khan, W.S.; Tahir, M.; Abid, M.; Idrees, F.; Butt, F.K.; Ali, Z.; Mahmood, N. The synergistic effect between WO3 and g-C3N4 towards efficient visible-light-driven photocatalytic performance. New J. Chem. 2014, 38, 5462–5469. [Google Scholar] [CrossRef]
- Katsumata, H.; Tachi, Y.; Suzuki, T.; Kaneco, S. Z-scheme photocatalytic hydrogen production over WO3/g-C3N4 composite photocatalysts. RSC Adv. 2014, 4, 21405–21409. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Idrees, F.; Cao, C.; Ahmed, R.; Butt, F.K.; Butt, S.; Tahir, M.; Tanveer, M.; Aslam, I.; Ali, Z. Novel nano-flowers of Nb2O5 by template free synthesis and enhanced photocatalytic response under visible light. Sci. Adv. Mater. 2015, 7, 1298–1303. [Google Scholar] [CrossRef]
- Idrees, F.; Hou, J.; Cao, C.; Butt, F.K.; Shakir, I.; Tahir, M.; Idrees, F. Template-free synthesis of highly ordered 3D-hollow hierarchical Nb2O5 superstructures as an asymmetric supercapacitor by using inorganic electrolyte. Electrochim. Acta 2016, 216, 332–338. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Satoshi, I.; Abdullah, A.Z.; Mohamed, A.R. Enhanced sunlight photocatalytic performance over Nb2O5/ZnO nanorod composites and the mechanism study. Appl. Catal. A 2014, 471, 126–135. [Google Scholar] [CrossRef]
- Hong, Y.; Li, C.; Zhang, G.; Meng, Y.; Yin, B.; Zhao, Y.; Shi, W. Efficient and stable Nb2O5 modified g-C3N4 photocatalyst for removal of antibiotic pollutant. Chem. Eng. J. 2016, 299, 74–84. [Google Scholar] [CrossRef]
- Huang, Q.-Z.; Wang, J.-C.; Wang, P.-P.; Yao, H.-C.; Li, Z.-J. In-situ growth of mesoporous Nb2O5 microspheres on g-C3N4 nanosheets for enhanced photocatalytic H2 evolution under visible light irradiation. Int. J. Hydrogen Energy 2017, 42, 6683–6694. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A Review of Direct Z-Scheme Photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Huang, Z.-F.; Song, J.; Wang, X.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Switching charge transfer of C3N4/W18O49 from type-II to Z-scheme by interfacial band bending for highly efficient photocatalytic hydrogen evolution. Nano Energy 2017, 40, 308–316. [Google Scholar] [CrossRef]
- Huang, Y.-T.; Cheng, R.; Zhai, P.; Lee, H.; Chang, Y.-H.; Feng, S.-P. Solution-Based Synthesis of Ultrasmall Nb2O5 Nanoparticles for Functional Thin Films in Dye-Sensitized and Perovskite Solar Cells. Electrochim. Acta 2017, 236, 131–139. [Google Scholar] [CrossRef]
- Passoni, L.C.; Siddiqui, M.R.H.; Steiner, A.; Kozhevnikov, I.V. Niobium peroxo compounds as catalysts for liquid-phase oxidation with hydrogen peroxide. J. Mol. Catal. A: Chem. 2000, 153, 103–108. [Google Scholar] [CrossRef]
- Narendar, Y.; Messing, G.L. Synthesis, decomposition and crystallization characteristics of peroxo− citrato− niobium: an aqueous niobium precursor. Chem. Mater. 1997, 9, 580–587. [Google Scholar] [CrossRef]
- Silva, G.; Carvalho, K.; Lopes, O.; Ribeiro, C. g-C3N4/Nb2O5 heterostructures tailored by sonochemical synthesis: Enhanced photocatalytic performance in oxidation of emerging pollutants driven by visible radiation. Appl. Catal. B Environ. 2017, 216, 70–79. [Google Scholar]
- Lopes, O.; Paris, E.; Ribeiro, C. Synthesis of Nb2O5 Nanoparticles Through the Oxidant Peroxide Method Applied to Organic Pollutant Photodegradation: A Mechanistic Study. Appl. Catal. B 2014, 144, 800–808. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. Niobium oxide solution chemistry. J. Raman Spectrosc. 1991, 22, 83–89. [Google Scholar] [CrossRef]
- Compton, O.C.; Osterloh, F.E. Niobate nanosheets as catalysts for photochemical water splitting into hydrogen and hydrogen peroxide. J. Phys. Chem. C 2008, 113, 479–485. [Google Scholar] [CrossRef]
- Nakajima, K.; Fukui, T.; Kato, H.; Kitano, M.; Kondo, J.N.; Hayashi, S.; Hara, M. Structure and Acid Catalysis of Mesoporous Nb2O5·nH2O. Chem. Mater. 2010, 22, 3332–3339. [Google Scholar] [CrossRef]
- Cui, Y.; Ding, Z.; Liu, P.; Antonietti, M.; Fu, X.; Wang, X. Metal-free activation of H2O2 by g-C3N4 under visible light irradiation for the degradation of organic pollutants. Phys. Chem. Chem. Phys. 2012, 14, 1455–1462. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulou, T.; Papailias, I.; Todorova, N.; Boukos, N.; Liu, Y.; Yu, J.; Trapalis, C. Tailoring the energy band gap and edges’ potentials of g-C3N4/TiO2 composite photocatalysts for NOx removal. Chem. Eng. J. 2017, 310, 571–580. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Dillert, R.; Robben, L.; Bahnemann, D.W. Photonic efficiency and mechanism of photocatalytic molecular hydrogen production over platinized titanium dioxide from aqueous methanol solutions. Catal. Today 2011, 161, 196–201. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Ivanova, I.; Bahnemann, D.W. Long-term investigation of the photocatalytic hydrogen production on platinized TiO2: An isotopic study. Energy Environ. Sci. 2014, 7, 1420–1425. [Google Scholar] [CrossRef]
- Chen, J.; Shen, S.; Guo, P.; Wang, M.; Wu, P.; Wang, X.; Guo, L. In-situ reduction synthesis of nano-sized Cu2O particles modifying g-C3N4 for enhanced photocatalytic hydrogen production. Appl. Catal. B 2014, 152–153, 335–341. [Google Scholar] [CrossRef]
- Chai, B.; Peng, T.; Mao, J.; Li, K.; Zan, L. Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys. 2012, 14, 16745–16752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Wu, G.; Chen, W. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production. Nanoscale 2012, 4, 5300–5303. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, L.; Zhuang, C.; Peng, T.; Li, R.; Li, X. Highly Asymmetric Phthalocyanine as a Sensitizer of Graphitic Carbon Nitride for Extremely Efficient Photocatalytic H2 Production under Near-Infrared Light. ACS Catal. 2014, 4, 162–170. [Google Scholar] [CrossRef]
- Chen, Z.; Sun, P.; Fan, B.; Zhang, Z.; Fang, X. In Situ Template-Free Ion-Exchange Process to Prepare Visible-Light-Active g-C3N4/NiS Hybrid Photocatalysts with Enhanced Hydrogen Evolution Activity. J. Phys. Chem. C 2014, 118, 7801–7807. [Google Scholar] [CrossRef]
- Liu, G.; Wang, T.; Zhang, H.; Meng, X.; Hao, D.; Chang, K.; Li, P.; Kako, T.; Ye, J. Nature-Inspired Environmental “Phosphorylation” Boosts Photocatalytic H2 Production over Carbon Nitride Nanosheets under Visible-Light Irradiation. Angew. Chem. Int. Ed. 2015, 54, 13561–13565. [Google Scholar] [CrossRef]
- Chen, X.; Yu, T.; Fan, X.; Zhang, H.; Li, Z.; Ye, J.; Zou, Z. Enhanced activity of mesoporous Nb2O5 for photocatalytic hydrogen production. Appl. Surf. Sci. 2007, 253, 8500–8506. [Google Scholar] [CrossRef]
- Sreethawong, T.; Ngamsinlapasathian, S.; Lim, S.H.; Yoshikawa, S. Investigation of thermal treatment effect on physicochemical and photocatalytic H2 production properties of mesoporous-assembled Nb2O5 nanoparticles synthesized via a surfactant-modified sol–gel method. Chem. Eng. J. 2013, 215–216, 322–330. [Google Scholar] [CrossRef]
- Pai, Y.-H.; Fang, S.-Y. Preparation and characterization of porous Nb2O5 photocatalysts with CuO, NiO and Pt cocatalyst for hydrogen production by light-induced water splitting. J. Power Sources 2013, 230, 321–326. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Yang, H.-C.; Wang, W.-L. Synthesis of mesoporous Nb2O5 photocatalysts with Pt, Au, Cu and NiO cocatalyst for water splitting. Catal. Today 2011, 174, 106–113. [Google Scholar] [CrossRef]
- Manuspiya, H. Electrical Properties of Niobium Based Oxides-Ceramics and Single Crystal Fibers Grown by the Laser-Heated Pedestal Growth (LHPG) Technique. Ph.D. Thesis, Penn State University, University Park, PA, USA, 4 April 2003. [Google Scholar]
- Gelderman, K.; Lee, L.; Donne, S.W. Flat-Band Potential of a Semiconductor: Using the Mott–Schottky Equation. J. Chem. Educ. 2007, 84, 685. [Google Scholar] [CrossRef]
- Mousa, G.; Golnaraghi, F.; DeVaal, J.; Young, A. Detecting proton exchange membrane fuel cell hydrogen leak using electrochemical impedance spectroscopy method. J. Power Sources 2014, 246, 110–116. [Google Scholar] [CrossRef]










| Photocatalysts | Co-Catalyst | Scavenger | Reaction Conditions a | Light Source | H2 (µmol/h/g) | Ref |
|---|---|---|---|---|---|---|
| Nb2O5 | Pt (1.0 wt %) | TEOA (10 Vol. %) | 45 mL, 10 mg (7 h) | Simulated Solar Lamp | 41,200 | Present |
| g-C3N4 | 33,460 | |||||
| P25 | 45,460 | |||||
| Nb2O5/g-C3N4 (Best Composite) | 110,000 | |||||
| Cu2O/g-C3N4-Mechanical Composite | Pt (1.0 wt %) | TEOA (10 Vol. %) | 180 mL, 100 mg (11 h) | Visible Light | 142 | [35] |
| Cu2O (0.05 wt %)-g-C3N4-In-Situ Composite | 241 | |||||
| g-C3N4 | 142 | |||||
| Pt-g-C3N4-TiO2 | Pt (not defined) | TEOA (10 Vol. %) | 100 mL, 100 mg | Visible Light (420 nm) | 1240 | [36] |
| g-C3N4-Pt-TiO2 | 1780 | |||||
| Urea-polymerized-g-C3N4 | Pt (3.0 wt %) | TEA (10 Vol. %) | 100 mL, 80 mg (8 h) | Simulated Solar Lamp | 590 | [37] |
| nil | 100 mL, 80 mg (25 h) | 3.13 | ||||
| Zn-tri-PcNc sensitized g-C3N4 | Pt (1.0 wt %) | TEOA (10 Vol. %) | 10 mL H2O + 50 mL Ascorbic Acid, 10 mg (10 h) | Infrared 500 nm | 12,500 | [38] |
| g-C3N4 | Pt (2.0 wt %) | TEOA (10 Vol. %) | 100 mL, 100 mg | Visible Light (420 nm) | 480 | [39] |
| NiS/g-C3N4 | NiS (1.5 mol %) | 450 | ||||
| g-C3N4 | Pt (3.0 wt %) | TEOA (10 Vol. %) + K2HPO4 | 270 mL, 50 mg | Visible Light (420 nm) | 18,940 | [40] |
| Nb2O5/g-C3N4 (Best Composite) | -- | Methanol (10 Vol. %) | 45 mL, 10 mg (7 h) | Simulated Solar Lamp | 5530 | Present |
| Pt (1.0 wt %) | 9322 | |||||
| Nb2O5/g-C3N4 (Physically Mixed) | 851 | |||||
| Mesoporous Nb2O5 | Pt (0.5 wt %) | Methanol (12.5 Vol. %) | 400 mL, 10 mg (7 h) | Mercury Lamp (200–600 nm) | 12,350 | [41] |
| Mesoporous Nb2O5 nano particles | Pt (1.0 wt %) | Methanol (9.1 Vol. %) | 220 mL, 20 mg (4 h) | Mercury Lamp Internal Irradiation | 191 | [42] |
| Mesoporous Nb2O5 | -- | Methanol (1M) + H2SO4 (1M) | 200 mL, 200 mg (6 h) | White Light Source | 1120 | [43] |
| Pt (2.0 wt %) | 510 | |||||
| CuO | 1405 | |||||
| NiO | 800 | |||||
| Mesoporous Nb2O5 | -- | MeOH: H2O = (1:5) | 550 mL, 200 mg (6 h) | UV Light (360 nm) Internal Irradiation | 328 | [44] |
| Au (1.0 wt %) | 2091 | |||||
| Pt (1.0 wt %) | 4647 | |||||
| Cu (1.0 wt %) | 1572 | |||||
| NiO (1.0 wt %) | 709 | |||||
| C | 8.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrees, F.; Dillert, R.; Bahnemann, D.; Butt, F.K.; Tahir, M. In-Situ Synthesis of Nb2O5/g-C3N4 Heterostructures as Highly Efficient Photocatalysts for Molecular H2 Evolution under Solar Illumination. Catalysts 2019, 9, 169. https://doi.org/10.3390/catal9020169
Idrees F, Dillert R, Bahnemann D, Butt FK, Tahir M. In-Situ Synthesis of Nb2O5/g-C3N4 Heterostructures as Highly Efficient Photocatalysts for Molecular H2 Evolution under Solar Illumination. Catalysts. 2019; 9(2):169. https://doi.org/10.3390/catal9020169
Chicago/Turabian StyleIdrees, Faryal, Ralf Dillert, Detlef Bahnemann, Faheem K. Butt, and Muhammad Tahir. 2019. "In-Situ Synthesis of Nb2O5/g-C3N4 Heterostructures as Highly Efficient Photocatalysts for Molecular H2 Evolution under Solar Illumination" Catalysts 9, no. 2: 169. https://doi.org/10.3390/catal9020169
APA StyleIdrees, F., Dillert, R., Bahnemann, D., Butt, F. K., & Tahir, M. (2019). In-Situ Synthesis of Nb2O5/g-C3N4 Heterostructures as Highly Efficient Photocatalysts for Molecular H2 Evolution under Solar Illumination. Catalysts, 9(2), 169. https://doi.org/10.3390/catal9020169