Aerobic Epoxidation of Low-Molecular-Weight and Polymeric Olefins by a Supramolecular Manganese Porphyrin Catalyst
Abstract
:1. Introduction
2. Results and Discussion
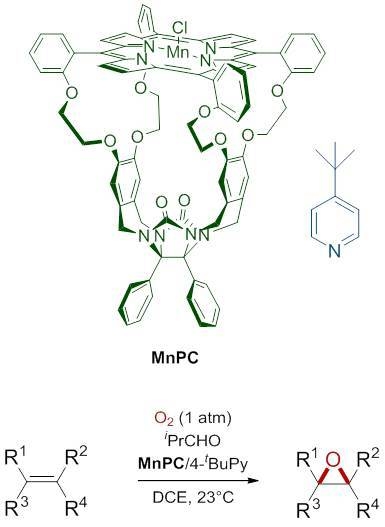
2.1. Reaction Optimization
2.2. Scope of the Aerobic Epoxidation of Olefins Catalyzed by Manganese Porphyrin Cage Compound MnPC
2.3. Aerobic Epoxidation of Polymers Catalyzed by Manganese Porphyrin Cage Catalyst MnPC
2.4. Plausible Mechanism for the Aerobic Epoxidation Catalyzed by Manganese Porphyrin Cage Catalyst MnPC
3. Materials and Methods
3.1. Experimental Section
3.2. Synthesis of Manganese Porphyrin Cage Catalyst Mn1
3.3. General Procedure for the Catalytic Epoxidation of Olefins to Epoxides
3.4. General Procedure for the Catalytic Epoxidation of Polyenes to Epoxides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Poulos, T.L. Heme Enzyme Structure and Function. Chem. Rev. 2014, 114, 3919–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and Functionalization of Porphyrins through Organometallic Methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Groves, J.T. Oxygen Activation and Radical Transformations in Heme Proteins and Metalloporphyrins. Chem. Rev. 2018, 118, 2491–2553. [Google Scholar] [CrossRef] [PubMed]
- Lua, H.; Zhang, X.P. Catalytic C–H Functionalization by Metalloporphyrins: Recent Developments and Future Directions. Chem. Soc. Rev. 2011, 40, 1899–1909. [Google Scholar] [CrossRef]
- Barona-Castaño, J.C.; Carmona-Vargas, C.C.; Brocksom, T.J.; De Oliveira, K.T. Porphyrins as Catalysts in Scalable Organic Reactions. Molecules 2016, 21, 310. [Google Scholar] [CrossRef]
- Van Dongen, S.F.M.; Elemans, J.A.A.W.; Rowan, A.E.; Nolte, R.J.M. Processive Catalysis. Angew. Chem. Int. Ed. 2014, 53, 11420–11428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thordarson, P.; Bijsterveld, E.J.A.; Rowan, A.E.; Nolte, R.J.M. Epoxidation of Polybutadiene by a Topologically Linked Catalyst. Nature 2003, 424, 915–918. [Google Scholar] [CrossRef]
- Coumans, R.G.E.; Elemans, J.A.A.W.; Nolte, R.J.M.; Rowan, A.E. Processive Enzyme Mimic: Kinetics and Thermodynamics of the Threading and Sliding Process. Proc. Natl. Acad. Sci. USA 2006, 103, 19647–19651. [Google Scholar] [CrossRef]
- Monnereau, C.; Ramos, P.H.; Deutman, A.B.C.; Elemans, J.A.A.W.; Nolte, R.J.M.; Rowan, A.E. Porphyrin Macrocyclic Catalysts for the Processive Oxidation of Polymer Substrates. J. Am. Chem. Soc. 2010, 132, 1529–1531. [Google Scholar] [CrossRef]
- De Torres, M.; van Hameren, R.; Nolte, R.J.M.; Rowan, A.E.; Elemans, J.A.A.W. Photocatalytic Oxidation of Stilbene by Self-Assembled Stacks of Manganese Porphyrins. Chem. Commun. 2013, 49, 10787–10789. [Google Scholar] [CrossRef]
- Pereira, M.M.; Dias, L.D.; Calvete, M.J.F. Metalloporphyrins: Bioinspired Oxidation Catalysts. ACS Catal. 2018, 8, 10784–10808. [Google Scholar] [CrossRef]
- Yamada, T.; Takai, T.; Rhode, O.; Mukaiyama, T. Direct Epoxidation of Olefins Catalyzed by Nickel(II) Complexes with Molecular Oxygen and Aldehydes. Bull. Chem. Soc. Jpn. 1991, 64, 2109–2117. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Imagawa, K.; Nagata, T.; Mukaiyama, T. Enantioselective Epoxidation of Unfunctionalized Olefins with Molecular Oxygen and Aldehyde Catalyzed by Optically Active Manganese(III) Complexes. Chem. Lett. 1992, 21, 2231–2234. [Google Scholar] [CrossRef]
- Nagata, T.; Imagawa, K.; Yamada, T.; Mukaiyama, T. Enantioselective Aerobic Epoxidation of Acyclic Simple Olefins Catalyzed by the Optically Active β-Ketoiminato Manganese(III) Complex. Chem. Lett. 1994, 23, 1259–1262. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, C.; Tanga, C.; Jiao, N. Recent Advances in Transition-Metal Catalyzed Reactions Using Molecular Oxygen as the Oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, P.F.; Otte, M.; Dürr, M.; Ivanović-Burmazović, I.; Reek, J.N.H.; de Bruin, B. A Self-Assembled Molecular Cage for Substrate-Selective Epoxidation Reactions in Aqueous Media. ACS Catal. 2016, 6, 3106–3112. [Google Scholar] [CrossRef]
- Farokhi, A.; Berijani, K.; Hosseini-Monfared, H. Manganes-Porphyrin as Efficient Enantioselective Catalyst for Aerobic Epoxidation of Olefins. Catal. Lett. 2018, 148, 2608–2618. [Google Scholar] [CrossRef]
- Berijani, K.; Farokhi, A.; Hosseini-Monfared, H.; Janiak, C. Enhanced Enantioselective Oxidation of Olefins Catalyzed by Mn-Porphyrin Immobilized on Graphene Oxide. Tetrahedron 2018, 74, 2202–2210. [Google Scholar] [CrossRef]
- Farokhi, A.; Hosseini-Monfared, H. Highly Efficient Asymmetric Epoxidation of Olefins with a Chiral Manganese-Porphyrin Covalently Bound to Mesoporous SBA-15: Support Effect. J. Catal. 2017, 352, 229–238. [Google Scholar] [CrossRef]
- For similar results see: Zhou, X.-T.; Tang, Q.-H.; Ji, H.-B. Remarkable Enhancement of Aerobic Epoxidation Reactivity for Olefins Catalyzed by µ-oxo-Bisiron(III) Porphyrins under Ambient Conditions. Tetrahedron Lett. 2009, 50, 6601–6605. [Google Scholar] [CrossRef]
- Zhou, X.-T.; Ji, H.-B.; Xu, J.-C.; Pei, L.-X.; Wang, L.-F.; Yao, X.-D. Enzymatic-like Mediated Olefins Epoxidation by Molecular Oxygen under Mild Conditions. Tetrahedron Lett. 2007, 48, 2691–2695. [Google Scholar] [CrossRef]
- Groves, J.T.; Nemo, T.E. Epoxidation Reactions Catalyzed by Iron Porphyrins. Oxygen Transfer from Iodosylbenzene. J. Am. Chem. Soc. 1983, 105, 5786–5791. [Google Scholar] [CrossRef]
- Battioni, P.; Renaud, J.P.; Bartoli, J.F.; Reina-Artiles, M.; Fort, M.; Mansuy, D. Monooxygenase-like Oxidation of Hydrocarbons by H2O2 Catalyzed by Manganese Porphyrins and Imidazole: Selection of the Best Catalytic System and Nature of the Active Oxygen Species. J. Am. Chem. Soc. 1988, 110, 8462–8470. [Google Scholar] [CrossRef]
- Aguiar, M.; Cabral de Menezes, S.; Akcelrud, L. Configurational Double Bond Selectivity in the Epoxidation of Hydroxy-terminated Polybutadiene with m-Chloroperbenzoic Acid. Macromol. Chem. Phys. 1994, 195, 3937–3948. [Google Scholar] [CrossRef]
- Gan, L.-H.; Ng, S.-C. Kinetic Studies of the Performic Acid Epoxidation of Natural Rubber Latex Stabilized by Cationic Surfactant. Eur. Polym. J. 1986, 22, 573–576. [Google Scholar] [CrossRef]
- Davies, C.K.L.; Wolfe, S.V.; Gelling, I.R.; Thomas, A.G. Strain Crystallization in Random Copolymers Produced by Epoxidation of cis 1,4-Polyisoprene. Polymer 1983, 24, 107–113. [Google Scholar] [CrossRef]
- Elemans, J.A.A.W.; Bijsterveld, E.J.A.; Rowan, A.E.; Nolte, R.J.M. A Host-Guest Epoxidation Catalyst with Enhanced Activity and Stability. Chem. Commun. 2000, 24, 2443–2444. [Google Scholar] [CrossRef]
- Nunes, G.S.; Mayer, I.; Toma, H.E.; Araki, K. Kinetics and Mechanism of Cyclohexane Oxidation Catalyzed by Supramolecular Manganese(III) Porphyrins. J. Catal. 2005, 236, 55–61. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Fujioka, N.; Kotani, H.; Ohkubo, K.; Lee, Y.-M.; Nam, W. Mechanistic Insights into Hydride-Transfer and Electron-Transfer Reactions by a Manganese(IV)–Oxo Porphyrin Complex. J. Am. Chem. Soc. 2009, 131, 17127–17134. [Google Scholar] [CrossRef]
- Baglia, R.A.; Zaragoza, J.P.T.; Goldberg, D.P. Biomimetic Reactivity of Oxygen-Derived Manganese and Iron Porphyrinoid Complexes. Chem. Rev. 2017, 117, 13320–13352. [Google Scholar] [CrossRef]
- Den Boer, D.; Li, M.; Habets, T.; Iavicoli, P.; Rowan, A.E.; Nolte, R.J.M.; Speller, S.; Amabilino, D.B.; De Feyter, S.; Elemans, J.A.A.W. Detection of Different Oxidation States of Individual Manganese Porphyrins during their Oxidation with Oxygen at a Solid/Liquid Interface. Nat. Chem. 2013, 5, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, P.J.; Varghese, S.; Bijsterveld, E.J.A.; Thordarson, P.; Elemans, J.A.A.W.; Rowan, A.E.; Nolte, R.J.M. A Double-Cavity-Containing Porphyrin Host as a Highly Stable Epoxidation Catalyst. Eur. J. Org. Chem. 2015, 5246–5253. [Google Scholar] [CrossRef]
- Groves, J.T.; Stern, M.K. Synthesis, Characterization, and Reactivity of Oxomanganese(IV) Porphyrin Complexes. J. Am. Chem. Soc. 1988, 110, 8628–8638. [Google Scholar] [CrossRef]
- Nam, W.; Kim, H.J.; Kim, S.H.; Ho, R.Y.N.; Valentine, J.S. Metal Complex-Catalyzed Epoxidation of Olefins by Dioxygen with Co-Oxidation of Aldehydes. A Mechanistic Study. Inorg. Chem. 1996, 35, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Smegal, J.A.; Schardt, B.C.; Hill, C.L. Isolation, Purification, and Characterization of Intermediate (Iodosylbenzene)metalloporphyrin Complexes from the (Tetraphenylporphinato)manganese(III)-Iodosyl-benzene Catalytic Hydrocarbon Functionalization System. J. Am. Chem. Soc. 1983, 105, 3510–3515. [Google Scholar] [CrossRef]
- Elemans, J.A.A.W.; Bijsterveld, E.J.A.; Rowan, A.E.; Nolte, R.J.M. Manganese Porphyrin Hosts as Epoxidation Catalysts—Activity and Stability Control by Axial Ligand Effects. Eur. J. Org. Chem. 2007, 5, 751–757. [Google Scholar] [CrossRef]
- Pereira, A.; Martín, C.; Maya, C.; Belderrain, T.R.; Pérez, P.J. An Effective Dual Copper- and Sulfide-Catalytic System for the Epoxidation of Aldehydes with Phenyldiazomethane. Adv. Synth. Catal. 2013, 355, 2942–2951. [Google Scholar] [CrossRef] [Green Version]





| Entry | Catalyst Loading (mol%) | Solvent | Conversion (%) b | Yield (%) c |
|---|---|---|---|---|
| 1 | 0 | DCM | 18 | 15 |
| 2 | 0.01 | DCM | 62 | 49 |
| 3 | 0.1 | DCM | 96 | 84 |
| 4 | 0.5 | DCM | >99 | 93 |
| 5 d | 0.5 | DCM | 31 | 24 |
| 6 e | 0.5 | DCM | 0 | 0 |
| 7 | 0.5 | DCE | >99 | 92 |
| 8 | 0.5 | MeCN | 89 | 83 |
| 9 | 0.5 | MeOH | 56 | 42 |
| 10 f | 0.5 | DCE | >99 | 91 |

| Entry | Substrate | Product | Time (h) | Yield (%) b | cis/trans Ratio b |
|---|---|---|---|---|---|
| 1 |  |  | 4 | 94 | |
| 2 |  |  | 4 | 89 | |
| 3 |  |  | 4 | 72 | |
| 4 |  |  | 4 4 | 91 86 c | 95:5 90:10 |
| 5 |  |  | 8 24 8 | 32 45 d 30 c | <1:99 <1:99 5:95 |
| 6 |  |  | 8 8 | 67 61 c | <1:99 3:97 |
| 7 |  |  | 24 8 | 48 89 c | |
| 8 |  |  | 4 | 96 | |
| 9 |  |  | 4 | 95 |
| Entry | Substrate | Catalyst | Conversion (%) b |
|---|---|---|---|
| 1 |  | MnPC MnTPPCl | 83 57 |
| polybutadiene A | |||
| 2 |  | MnPC MnTPPCl | 76 52 |
| polybutadiene B | |||
| 3 |  | MnPC MnTPPCl | 79 54 |
| polyisoprene |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernar, I.; Rutjes, F.P.J.T.; Elemans, J.A.A.W.; Nolte, R.J.M. Aerobic Epoxidation of Low-Molecular-Weight and Polymeric Olefins by a Supramolecular Manganese Porphyrin Catalyst. Catalysts 2019, 9, 195. https://doi.org/10.3390/catal9020195
Bernar I, Rutjes FPJT, Elemans JAAW, Nolte RJM. Aerobic Epoxidation of Low-Molecular-Weight and Polymeric Olefins by a Supramolecular Manganese Porphyrin Catalyst. Catalysts. 2019; 9(2):195. https://doi.org/10.3390/catal9020195
Chicago/Turabian StyleBernar, Ivan, Floris P.J.T. Rutjes, Johannes A.A.W. Elemans, and Roeland J.M. Nolte. 2019. "Aerobic Epoxidation of Low-Molecular-Weight and Polymeric Olefins by a Supramolecular Manganese Porphyrin Catalyst" Catalysts 9, no. 2: 195. https://doi.org/10.3390/catal9020195
APA StyleBernar, I., Rutjes, F. P. J. T., Elemans, J. A. A. W., & Nolte, R. J. M. (2019). Aerobic Epoxidation of Low-Molecular-Weight and Polymeric Olefins by a Supramolecular Manganese Porphyrin Catalyst. Catalysts, 9(2), 195. https://doi.org/10.3390/catal9020195