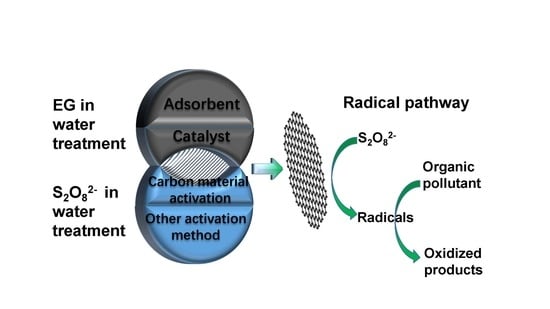
Preparation and Catalytic Performance of Expanded Graphite for Oxidation of Organic Pollutant
Abstract
:1. Introduction
2. Results and Discussion
2.1. Materials Characterization
2.2. Decolorization of AR97 in Different Systems
2.3. The Adsorptive and Catalytic Performances of Different EG Samples
2.4. Effect of EG Dosage and PDS Concentration on AR97 Removal
2.5. Effect of Initial pH
2.6. Reuse of EG
2.7. Quenching Experiments and Radical Mechanism
2.8. Electron Paramagnetic Resonance (EPR) Studies
2.9. Verification of Method Reproducibility by Commercial Expandable Graphite
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Expanded Graphites
3.3. Catalytic Degradation Experimental and Analytical Methods
3.4. Characterization of EG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Bouchard, J.; Maine, C.; Argyropoulos, D.S.; Berry, R.M. Kraft Pulp Bleaching Using In-situ Dimethyldioxirane: Mechanism and Reactivity of the Oxidants. Holzforschung—Int. J. Biol. Chem. Phys. Technol. Wood 1998, 52, 499–505. [Google Scholar]
- Wang, S. A Comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dyes Pigm. 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Hammouda, S.B.; Zhao, F.; Safaei, Z.; Srivastava, V.; Ramasamy, D.L.; Iftekhar, S.; Kalliola, S.; Sillanpää, M. Degradation and mineralization of phenol in aqueous medium by heterogeneous monopersulfate activation on nanostructured cobalt based-perovskite catalysts ACoO3 (A = La, Ba, Sr and Ce): Characterization, kinetics and mechanism study. Appl. Catal. B Environ. 2017, 215, 60–73. [Google Scholar] [CrossRef]
- Tan, C.; Gao, N.; Chu, W.; Li, C.; Templeton, M.R. Degradation of diuron by persulfate activated with ferrous ion. Sep. Purif. Technol. 2012, 95, 44–48. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Yang, L.; Lan, Y. Degradation of methyl orange by sodium persulfate activated with zero-valent zinc. Sep. Purif. Technol. 2014, 132, 168–173. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Zhang, J.; Quan, G.; Lan, Y. Efficient Degradation of Congo Red by Sodium Persulfate Activated with Zero-Valent Zinc. Water Air Soil Pollut. 2014, 225, 2121–2128. [Google Scholar] [CrossRef]
- Rodriguez, S.; Vasquez, L.; Romero, A.; Santos, A. Dye Oxidation in Aqueous Phase by Using Zero-Valent Iron as Persulfate Activator: Kinetic Model and Effect of Particle Size. Ind. Eng. Chem. Res. 2014, 53, 12288–12294. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Chen, C.; Duan, X.; Sun, H.; Wang, S. Synthesis of Magnetic Carbon Supported Manganese Catalysts for Phenol Oxidation by Activation of Peroxymonosulfate. Catalysts 2017, 7, 3. [Google Scholar] [CrossRef]
- Stoia, M.; Muntean, C.; Militaru, B. MnFe2O4 nanoparticles as new catalyst for oxidative degradation of phenol by peroxydisulfate. J. Environ. Sci. China 2017, 53, 269–277. [Google Scholar] [CrossRef]
- Zhao, Y.S.; Sun, C.; Sun, J.Q.; Zhou, R. Kinetic modeling and efficiency of sulfate radical-based oxidation to remove p-nitroaniline from wastewater by persulfate/Fe3O4 nanoparticles process. Sep. Purif. Technol. 2015, 142, 182–188. [Google Scholar] [CrossRef]
- Wang, Y.; Indrawirawan, S.; Duan, X.; Sun, H.; Ang, H.M.; Tadé, M.O.; Wang, S. New insights into heterogeneous generation and evolution processes of sulfate radicals for phenol degradation over one-dimensional α-MnO2 nanostructures. Chem. Eng. J. 2015, 266, 12–20. [Google Scholar] [CrossRef]
- Saputra, E.; Muhammad, S.; Sun, H.; Wang, S. Activated carbons as green and effective catalysts for generation of reactive radicals in degradation of aqueous phenol. RSC Adv. 2013, 3, 21905–21910. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Kang, J.; Wang, Y.; Indrawirawan, S.; Wang, S. Insights into Heterogeneous Catalysis of Persulfate Activation on Dimensional-Structured Nanocarbons. ACS Catal. 2015, 5, 4629–4636. [Google Scholar] [CrossRef]
- Chen, H.; Carroll, K.C. Metal-free catalysis of persulfate activation and organic-pollutant degradation by nitrogen-doped graphene and aminated graphene. Environ. Pollut. 2016, 215, 96–102. [Google Scholar] [CrossRef]
- Sun, H.; Liu, S.; Zhou, G.; Ang, H.M.; Tadé, M.O.; Wang, S. Reduced graphene oxide for catalytic oxidation of aqueous organic pollutants. ACS Appl. Mater. Interfaces 2012, 4, 5466–5471. [Google Scholar] [CrossRef]
- Xin, C.; Guo, H.; Zhang, Y.; Xiao, W.; Yang, L. Non-photochemical production of singlet oxygen via activation of persulfate by carbon nanotubes. Water Res. 2017, 113, 80–88. [Google Scholar]
- Lee, H.; Lee, H.J.; Jeong, J.; Lee, J.; Park, N.B.; Lee, C. Activation of persulfates by carbon nanotubes: Oxidation of organic compounds by nonradical mechanism. Chem. Eng. J. 2015, 266, 28–33. [Google Scholar] [CrossRef]
- Duan, X.; Su, C.; Zhou, L.; Sun, H.; Suvorova, A.; Odedairo, T.; Zhu, Z.; Shao, Z.; Wang, S. Surface controlled generation of reactive radicals from persulfate by carbocatalysis on nanodiamonds. Appl. Catal. B Environ. 2016, 194, 7–15. [Google Scholar] [CrossRef]
- Matzek, L.W.; Carter, K.E. Activated persulfate for organic chemical degradation: A review. Chemosphere 2016, 151, 178–188. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, J.; Han, Y.; Zhu, J.; Zhou, L.; Lan, Y. Insights into the mechanism of persulfate activated by rice straw biochar for the degradation of aniline. Chemosphere 2018, 200, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Duan, X.; Wang, C.; Sun, H.; Tan, X.; Tade, M.O.; Wang, S. Nitrogen-doped bamboo-like carbon nanotubes with Ni encapsulation for persulfate activation to remove emerging contaminants with excellent catalytic stability. Chem. Eng. J. 2018, 332, 398–408. [Google Scholar] [CrossRef]
- Liu, N.; Zhang, L.; Xue, Y.; Lv, J.; Yu, Q.; Yuan, X. Nitrogen-Doped Carbon Material as a Catalyst for the Degradation of Direct Red23 based on Persulfate Oxidation. Sep. Purif. Technol. 2017, 184, 213–219. [Google Scholar] [CrossRef]
- Guo, Y.; Zeng, Z.; Zhu, Y.; Huang, Z.; Cui, Y.; Yang, J. Catalytic oxidation of aqueous organic contaminants by persulfate activated with sulfur-doped hierarchically porous carbon derived from thiophene. Appl. Catal. B Environ. 2018, 220, 635–644. [Google Scholar] [CrossRef]
- Chung, D.D.L. A review of exfoliated graphite. J. Mater. Sci. 2016, 51, 554–568. [Google Scholar] [CrossRef]
- Raulo, A.; Suin, S.; Paria, S.; Khatua, B.B. Expanded graphite (EG) as a potential filler in the reduction of percolation threshold of multiwall carbon nanotubes (MWCNT) in the PMMA/HDPE/EG/MWCNT nanocomposites. Polym. Compos. 2016, 37, 2070–2082. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S. The determination of the adsorption performance of graphite for VOCs and formaldehyde. Energy Build. 2012, 46, 56–61. [Google Scholar] [CrossRef]
- Hanifah, M.F.R.; Jaafar, J.; Aziz, M.; Ismail, A.F.; Rahman, M.A.; Othman, M.H.D. Synthesis of Graphene Oxide Nanosheets via Modified Hummers’ Method and Its Physicochemical Properties. J. Teknol. 2015, 74, 195–198. [Google Scholar] [CrossRef]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye Adsorption on Layered Graphite Oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Jr, W.S.H.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Duan, X.; Sun, H.; Ao, Z.; Zhou, L.; Wang, G.; Wang, S. Unveiling the active sites of graphene-catalyzed peroxymonosulfate activation. Carbon 2016, 107, 371–378. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.Y.; Kim, K.S.; Jin, F.L. A novel drying process for oil adsorption of expanded graphite. Carbon Lett. 2013, 14, 193–195. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Jin, C.; Deng-Xin, L.I. Adsorption of Cr (VI) in Wastewater on Expanded Graphite. Environ. Sci. Technol. 2012, 35, 149–152. [Google Scholar]
- Li, J.T.; Song, Y.L.; Meng, X.Y.; Liu, C.M. Decolourization of C.I. Direct blue 78 aqueous solution in presence of exfoliated graphite under ultrasound irradiation. Indian J. Chem. Technol. 2009, 16, 411–416. [Google Scholar]
- Poh, H.L.; Sanek, F.; Ambrosi, A.; Zhao, G.; Sofer, Z.; Pumera, M. Graphenes prepared by Staudenmaier, Hofmann and Hummers methods with consequent thermal exfoliation exhibit very different electrochemical properties. Nanoscale 2012, 4, 3515–3522. [Google Scholar] [CrossRef]
- Peng, T.; Liu, B.; Gao, X.; Luo, L.; Sun, H. Preparation, Quantitative Surface Analysis, Intercalation Characteristics and Industrial Implications of Low Temperature Expandable Graphite. Appl. Surf. Sci. 2018, 444, 800–810. [Google Scholar] [CrossRef]
- Shi, P.; Su, R.; Zhu, S.; Zhu, M.; Li, D.; Xu, S. Supported cobalt oxide on graphene oxide: highly efficient catalysts for the removal of Orange II from water. J. Hazard. Mater. 2012, 229–230, 331–339. [Google Scholar] [CrossRef]
- Hassouna, F.; Laachachi, A.; Chapron, D.; Mouedden, Y.E.; Toniazzo, V.; Ruch, D. Development of new approach based on raman spectroscopy to study the dispersion of expanded graphite in poly (lactide). Polym. Degrad. Stab. 2011, 96, 2040–2047. [Google Scholar] [CrossRef]
- Tang, L.; Liu, Y.; Wang, J.; Zeng, G.; Deng, Y.; Dong, H.; Feng, H.; Wang, J.; Peng, B. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism. Appl. Catal. B Environ. 2018, 231, 1–10. [Google Scholar] [CrossRef]
- Vermisoglou, E.C.; Giannakopoulou, T.; Romanos, G.E.; Boukos, N.; Giannouri, M.; Lei, C.; Lekakou, C.; Trapalis, C. Non-activated high surface area expanded graphite oxide for supercapacitors. Appl. Surf. Sci. 2015, 358, 110–121. [Google Scholar] [CrossRef]
- Pang, X.Y.; Tian, Y.; Weng, M.Q. Preparation of expandable graphite with silicate assistant intercalation and its effect on flame retardancy of ethylene vinyl acetate composite. Polym. Compos. 2015, 36, 1407–1416. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Lekuan, M.A.; Fan, P.; Congbin, X.U.; Jiao, C.; Lin, A. Preparation of Composite Modified Expanded Graphite and Its Adsorption on Acid Brilliant Blue Dye. Chem. J. Chin. Univ. 2016, 37, 335–341. [Google Scholar]
- Yang, S.; Li, L.; Xiao, T.; Zhang, Y.; Zheng, D. Promoting effect of ammonia modification on activated carbon catalyzed peroxymonosulfate oxidation. Sep. Purif. Technol. 2016, 160, 81–88. [Google Scholar] [CrossRef]
- Zhang, J.; Shao, X.; Shi, C.; Yang, S. Decolorization of Acid Orange 7 with peroxymonosulfate oxidation catalyzed by granular activated carbon. Chem. Eng. J. 2013, 232, 259–265. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Shao, X.; Niu, R.; Wang, L. Activated carbon catalyzed persulfate oxidation of Azo dye acid orange 7 at ambient temperature. J. Hazard. Mater. 2011, 186, 659–666. [Google Scholar] [CrossRef]
- Wang, Y.; Ao, Z.; Sun, H.; Duan, X.; Wang, S. Activation of peroxymonosulfate by carbonaceous oxygen groups: Experimental and density functional theory calculations. Appl. Catal. B Environ. 2016, 198, 295–302. [Google Scholar] [CrossRef]
- Li, M.; Hsieh, T.C.; Doong, R.A.; Huang, C.P. Tuning the adsorption capability of multi-walled carbon nanotubes to polar and non-polar organic compounds by surface oxidation. Sep. Purif. Technol. 2013, 117, 98–103. [Google Scholar] [CrossRef]
- Wu, W.; Chen, W.; Lin, D.; Yang, K. Influence of surface oxidation of multiwalled carbon nanotubes on the adsorption affinity and capacity of polar and nonpolar organic compounds in aqueous phase. Environ. Sci. Technol. 2012, 46, 5446–5454. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, Z.; Yang, J.; Cui, Y.; Wu, A.; Li, Z.; Huang, Z. Degradation of Phenol with Persulfate Activated by Surface Modified Activated Carbon. Chem. J. Chin. Univ. 2017, 38, 1241–1248. [Google Scholar]
- Yu, X.; Bao, Z.; Barker, J.R. Free Radical Reactions Involving Cl, Cl2·− and SO4·− in the 248 nm Photolysis of Aqueous Solutions Containing S2O82− and Cl−. J. Phys. Chem. A 2004, 108, 295–308. [Google Scholar] [CrossRef]
- Wang, Z.M.; Huang, T.Y.; Chen, J.B.; Li, W.W.; Zhang, L.M. Degradation of Acid Orange 7 with Persulfate Activated by Silver Loaded Granular Activated Carbon. Environ. Sci. 2015, 36, 4127–4134. [Google Scholar]
- Georgi, A.; Kopinke, F.D. Interaction of adsorption and catalytic reactions in water decontamination processes: Part I. Oxidation of organic contaminants with hydrogen peroxide catalyzed by activated carbon. Appl. Catal. B Environ. 2005, 58, 9–18. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, Y.; Kang, J.; Wang, S. N-Doping-Induced Nonradical Reaction on Single-Walled Carbon Nanotubes for Catalytic Phenol Oxidation. Acs Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (·OH/·O−) in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef]
- And, M.E.L.; Tarr, M.A. Inhibition of Hydroxyl Radical Reaction with Aromatics by Dissolved Natural Organic Matter. Environ. Sci. Technol. 2000, 34, 444–449. [Google Scholar]
- Ziajka, J.; Pasiuk-Bronikowska, W. Rate constants for atmospheric trace organics scavenging SO4·− in the Fe-catalysed autoxidation of S (IV). Atmos. Environ. 2005, 39, 1431–1438. [Google Scholar] [CrossRef]
- Li, S.; Tian, S.; Feng, Y.; Lei, J.; Wang, P.; Xiong, Y. A comparative investigation on absorption performances of three expanded graphite-based complex materials for toluene. J. Hazard. Mater. 2010, 183, 506–511. [Google Scholar] [CrossRef]
- Ling, Z.; Yong, W.; Jin, S.W.; Lu, Q.Z.; Jiang, J. Adsorption isotherm, kinetic and mechanism of expanded graphite for sulfadiazine antibiotics removal from aqueous solutions. Environ. Technol. 2017, 38, 1–10. [Google Scholar]
- Pang, X.Y.; Lv, P.; Feng, Y.Q.; Liu, X. Study on the adsorbing characteristics of expanded graphite for organic dyes. Environ. Sci. 2008, 3, 150–157. [Google Scholar]
- Ouyang, D.; Yan, J.; Qian, L.; Chen, Y.; Han, L.; Su, A.; Zhang, W.; Ni, H.; Chen, M. Degradation of 1,4-dioxane by biochar supported nano magnetite particles activating persulfate. Chemosphere 2017, 184, 609–617. [Google Scholar] [CrossRef]
- Liang, C.; Huang, C.F.; Mohanty, N.; Kurakalva, R.M. A rapid spectrophotometric determination of persulfate anion in ISCO. Chemosphere 2008, 73, 1540–1543. [Google Scholar] [CrossRef]
- Morales-Torres, S.; Silva, A.M.T.; Maldonado-Hódar, F.J.; Machado, B.F.; Pérez-Cadenas, A.F.; Faria, J.L.; Figueiredo, J.L.; Carrasco-Marín, F. Pt-catalysts supported on activated carbons for catalytic wet air oxidation of aniline: Activity and stability. Appl. Catal. B Environ. 2011, 105, 86–94. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, R.; Su, W.; Li, J. Preparation and Catalytic Performance of Expanded Graphite for Oxidation of Organic Pollutant. Catalysts 2019, 9, 280. https://doi.org/10.3390/catal9030280
Lan R, Su W, Li J. Preparation and Catalytic Performance of Expanded Graphite for Oxidation of Organic Pollutant. Catalysts. 2019; 9(3):280. https://doi.org/10.3390/catal9030280
Chicago/Turabian StyleLan, Ruijia, Wenbin Su, and Jitai Li. 2019. "Preparation and Catalytic Performance of Expanded Graphite for Oxidation of Organic Pollutant" Catalysts 9, no. 3: 280. https://doi.org/10.3390/catal9030280
APA StyleLan, R., Su, W., & Li, J. (2019). Preparation and Catalytic Performance of Expanded Graphite for Oxidation of Organic Pollutant. Catalysts, 9(3), 280. https://doi.org/10.3390/catal9030280