1. Introduction
Pharmaceutical compounds are an important part of toxic materials in wastewater that are currently growing around the world [
1]. They have been frequently detected in the aqueous medium, supposing a growing environmental problem for the scientific community. The excessive drug production demanded and consumed by humans and animals usually ends up in surface water [
1], sewage effluents [
2], groundwater [
3], and even drinking water [
4] due to the discharges from municipal wastewater treatment plants (WWTPs) [
1], and, consequently, these contaminants can lead to devastating effects on the environment [
3,
5]. Particularly, non-steroidal anti-inflammatory drugs (NSAIDs) have been found in water effluents at increasing concentrations and their removal from the environment is becoming a real challenge [
6]. In this work, naproxen (NAP) has been selected due to its presence in many kinds of water environments [
7,
8,
9], such as WWTPs effluents, surface water, and hospital wastewater effluent, in significant concentrations (<0.1 ng/L to 0.220 mg/L) [
2,
7,
8,
9,
10]. Despite the low values found (from ng/L to mg/L), the risk assessment associated with the risk quotient (RQ) of NAP has been reported in 84 fish, which is considered as a very high value, and consequently, demands the development of efficient alternative treatments of aqueous matrices containing NAP [
10]. Moreover, the toxicological studies demonstrated that NAP and their degradation byproducts exhibited adverse effects on aquatic organisms at an EC
50 value of 0.33 mg/L on
C. dubia [
11]. In addition, the NAP molecule is highly stable at neutral and basic pH media and its dissociation only occurs when it is heated in concentrated acid sulfuric/nitric solutions [
12,
13]. These properties make it difficult to be degraded in the conventional WWTPs that usually work near the neutral pH. As a consequence, NAP would pass through these conventional treatment processes and end up in the water environment [
14]. In this sense, the effectiveness of the removal of NAP from wastewater by different treatments, such as adsorption in activated process, biological degradation, photolysis, ozonation/H
2O
2, and adsorption on activated carbon, were 50–80%, no data, 99–100%, 98%, and 52%, respectively [
2]. In 2013, Ma et al. [
15] demonstrated that the generated derivate products by photo-degradation of NAP were more toxic than the parent compound. Accordingly, research on methods of NAP removal is becoming an interesting challenge, especially if the resulting derivate products are less toxic and more biodegradable than NAP [
2].
Catalytic wet peroxide oxidation (CWPO) is an advanced oxidation process where the heterogeneous catalyst catalyzes the decomposition of hydrogen peroxide into oxygen-based radicals at mild conditions of temperature and pressure [
16]. The reactive species (
•OH) are able to degrade even the most recalcitrant organic molecules and convert them into less persistent and non-contaminant compounds [
16]. CWPO was demonstrated to be effective for the removal of refractory organic compounds, traceable organic contaminants, or some inorganic pollutants, or in particular cases, to increase the wastewater biodegradability as a pretreatment of a subsequent biological treatment stage [
1,
17,
18,
19]. Heterogeneous CWPO reactions using magnetite (Fe
3O
4) supported on multi-walled carbon nanotubes (MWCNTs) as a catalyst were reported for the effective removal of methylene blue [
20], sulfamethazine [
21], 17α-methyltestosterone [
22], and atrazine [
23]. Furthermore, Fe
3O
4/MWCNTs material has been reported to show interesting properties such as the wide-range pH of work, stability, recyclability, and low toxicity towards the medium, making this catalyst an effective alternative for the treatment of different toxic compounds in wastewater. It is noteworthy to say that MWCNTs acting as catalytic support plays an important role for the dispersion of the magnetite into the surface, obtaining a minimum leaching of the phase active and allowing it to work at a wider pH range [
20,
21,
22,
23,
24,
25]. Indeed, the surface of the MWCNTs has been reported to be chemically functionalized to potentially enhance the density of the active sites responsible for the
•OH generation, promoting the
•OH species formation [
25]. In addition, MWCNTs are also known to be relatively stable in extreme environments, maintaining their mechanical properties along the process [
25]. Therefore, Fe
3O
4/MWCNTs catalysts can be more effective in the degradation of pollutants compared to those immobilized on carriers, where the separation and recovering of the solids is needed after the treatment. In this context, Fe
3O
4/MWCNTs material is an effective alternative for the easy separation of the solid from the aqueous solution by using a magnet [
23,
26]. The synthesis of the catalyst was assessed following the combination of two known methods (co-precipitation and hydrothermal method) [
27]. To the best of the authors’ knowledge, this is the first work based on the synthesis of a magnetic material (Fe
3O
4/MWCNTs) used in CWPO reactions for effective NAP degradation. The catalyst was also tested in different aqueous matrices spiked with the pollutant.
This study has been structured in four stages. The first stage was to follow a facile synthesis route of the catalyst based on the incorporation of magnetite on the surface of MWCNTs, obtaining the magnetic solid further used in the CWPO experiments. The morphological structure of the material was studied by transmission electron microscopy (TEM). Moreover, the textural and surface-chemical properties of the catalyst were full explored. Our second aim was to evaluate the effect of the operating parameters (H2O2 dose, pH, and temperature) on the CWPO process for the removal of NAP by using a response surface methodology (RSM) based on the Box–Behnken Design (BBD), allowing us to determine the optimal experimental conditions. As a third aim, the kinetics of the catalytic degradation of the drug was explored, the activation energy was calculated, and a degradation mechanism of the pollutant was proposed. In addition, the reusability and recyclability of the catalyst were evaluated. Finally, our fourth aim was to test the optimum CWPO process in three real aqueous matrices—a wastewater treatment plant effluent (WWTP), surface water (SW), and a hospital wastewater (HW) spiked with NAP—determining the total organic carbon (TOC) removal, the efficiency of H2O2, and the toxicity of the effluent after 8 h reaction time.
3. Materials and Methods
3.1. Materials
Multi-walled carbon nanotubes (MWCNTs) (diameter of 30–50 nm; length of 20 mm) were supplied by Sun Nanotech Co. Ltd. in Beijing, China. FeCl3·6H2O, FeCl2·4H2O, ammonia solution 25%, and H2O2 solution 30% (w/w) were purchased from Sigma-Aldrich (Overijse, Belgium). Naproxen (NAP) as sodium salt with a purity of more than 98% was purchased from Sigma-Aldrich. All the solutions used in the experiments were prepared in ultrapure water. Furthermore, a mini magnetic stirrer with plastic cover and an Aitsite magnet NFD 60 for the separation of the solid after reaction were acquired.
3.2. Synthesis of the Catalyst
3.2.1. Functionalization of the Support (MWCNTs)
An oxidative treatment of MWCNTs was carried out by using H
2O
2 as the oxidizing agent [
53]. According to the method of Singer et al. [
53] with some modifications, a solution of H
2O
2 18% (
w/w) was used to dissolve 2.0 g of MWCNTs. Then, the suspension was heated at 80 °C for 4 h and, after, was thoroughly filtered and washed with ultrapure water until the washing water reached neutral pH. The material obtained was dried in an oven at 100 °C for 10 h.
3.2.2. Preparation of the Catalyst (Fe3O4/MWCNTs)
The catalyst preparation was accomplished following the combination of two methods (co-precipitation and hydrothermal treatment) [
23], with some modifications in the composition of the catalyst. Briefly, 120 mL of ultrapure water containing 0.20 g of MWCNTs was vigorously stirred at 60 °C in a three-neck flask under the purge of nitrogen gas. Then, 0.28 g of FeCl
3·6H
2O, 0.08 g of FeCl
2·4H
2O, and 0.5 mL of ammonia solution (25%) were added. After stirring for 30 min, the Fe
3O
4/MWCNTs colloidal solution was formed. The obtained colloidal solution was transferred and sealed into a Teflon-lined autoclave reactor and then kept at 120 °C for 12 h. After, the precipitate was separated from the aqueous liquid by a magnet and then washed with ultrapure water until the washing water reached a pH value of 6.3. Finally, the formed solid of Fe
3O
4/MWCNTs nanocomposites were ultra-sonicated for 3 min. Then, they were dried in a vacuum oven at 60 °C for 24 h.
3.2.3. Support and Catalyst Characterization
The morphology of the catalyst and the distribution of the magnetic nanoparticles supported on the MWCNTs were studied using transmission electron microscope (TEM, JEOL 3000F, Peabody, MA, USA). The thermogravimetric analyses were carried out in a thermal analyzer TGA Q500 (STA 6000, PerkinElmer, Waltham, MA, USA) under air atmosphere, following a heating rate of 10 °C min−1 from 30 to 1000 °C. The Fourier-transform infrared spectra were recorded in a Thermo Nicolet F-TIR spectrophotometer (Thermo Fisher Scienctific, Waltham, MA, USA), in a wavelength range from 400 to 4000 cm−1. The porosity of the materials was studied by N2 adsorption–desorption isotherms at 77 K in an ASAP 2020 apparatus, with the samples outgassed for 3 h at 250 °C before the measurement. The specific surface area of the solids (SBET) was calculated using the Brunauer–Emmett–Teller (BET) equation and the micropore volume (VMic) was estimated by using Dubinin–Radushkevich equation. The elemental microanalysis was accomplished in a LECO CHNS-932 analyzer (Leco Corporation, St. Joseph, MI, USA), where 0.6–1.6 mg of sample was held in a combustion furnace at 1000 °C. The magnetic properties of the solids were determined in a MPMS 5S superconducting quantum interference device (SQUID, San Diego, CA, USA). The total iron content of the samples was measured by wavelength dispersive X-ray fluorescence analysis (WDXRF), using an Aχios spectrometer (PANalytical) equipped with a Rh anode X-ray tube and maximum power of 4 kW.
3.3. Catalytic Wet Peroxide Oxidation (CWPO) Tests
A typical-batch CWPO experiment was carried out in a three-neck round-bottom flask using magnetic stirring, where 130 mL of NAP solution (C0 = 10 mg/L) was added. The reactor had a reflux condenser and was maintained at a constant temperature using a thermostatic bath. pH was adjusted to the required value (with 1 M sulfuric acid solution) after the solution reached the reaction temperature; then, 0.13 g of catalyst, and immediately after, the required H2O2 dose were added, with time considered as zero for the catalytic reaction. At regular time intervals, samples were collected from the reactor and immediately filtered through a 0.45 µm PTFE filter.
When the CWPO experiment finished, the catalyst was separated from the reaction medium by the action of a magnet and the treated effluent was filtrated. The catalyst was washed several times with ultrapure water and dried before being used in the next cycle. The reusability of the catalyst was investigated during 3 consecutive tests at 50 °C, 2.5 mM of H2O2 dose, and 1.0 g/L of catalyst.
3.4. Statistical Analysis through Response Surface Methodology: Box–Behnken Design
In this work, the effect of the operation parameters on the catalytic degradation of NAP was investigated. The statistical design was carried out by testing three factors—temperature, initial pH, and H2O2 dose, in order to determine the optimum removal of NAP. In the experiments, the catalyst concentration was maintained constant in a value of 1 g/L, and the initial concentration of the pollutant was established in 10 mg/L, as it was optimized in previous studies on diclofenac removal by CWPO.
Box—Behnken Design (BBD) is a response surface methodology (RSM) based on three-level incomplete factorial designs [
54]. The coded levels used in BBD can be seen in
Table 9. In this study, only 16 experiments were needed, including four replicates (See
Table 3).
RMS methodology comprises a group of mathematical and statistical methods based on the fitting of empirical models to the experimental data acquired for the test design. Thus, RMS was applied to the experimental data using the commercial software Minitab [
55]. In addition, multiple linear regression analysis of the experimental data followed by F-test lack of fit and other tests were performed in order to select the best model.
A manual regression method was used to fitting the quadratic polynomial Equation (16) to the experimental data [
54]:
where
γ is the removal of NAP (%),
β0 is a fixed coefficient,
βi,
βii, and
βij are the coefficients for the linear, quadratic, and interaction effects, and
Xi and
Xj are the coded values of the independent input variables.
The effect of temperature (30, 50, and 70 °C) on the CWPO reaction using a H2O2 dose of 1.5 mM, a pH value of 5, and 1 g/L of catalyst was studied, determining the reaction kinetics of the NAP degradation.
3.5. Analytical Methods
NAP concentration was analyzed by High-Performance Liquid Chromatography, HPLC UV-Vis (Varian ProStar, Palo Alto, CA, USA), using a Perkin Elmer column (250 × 4.6 mm i.d., 5 µm) as stationary phase. The analyses were performed at 274 nm, using 50%/50% (v/v) of acetonitrile/acidified water solution (0.1% H3PO4) as mobile phase (0.5 mL/min) and a loop volume of 20 µL.
H
2O
2 concentration was measured at a wavelength of 410 nm using a UV-Vis spectrophotometer (Lambda 35 PerkinElmer, Waltham, MA, USA) after adding titanium (IV) oxysulfate solution to the sample. Finally, the real-water aqueous matrices were characterized by the measurement of the total organic carbon (TOC) and the total nitrogen (TN) concentrations with a TOC analyzer (Shimadzu TOC VSCH, Kioto, Japan), and the chemical oxygen demand (COD), conductivity, suspended solids concentration, aromaticity, phenolic compounds, and nitrate (NO
3−) ions were measured according to the Standard Methods [
56].
3.6. Toxicity Tests
The internationally standardized aquatic ecotoxicity test (Microtox M500 Analyzer, Carlsbad, CA, USA) was applied to measure the toxicity of the samples before and after the CWPO treatment. The test is based on the inhibition of the light emission by the marine bacterium
Vibrio Fischeri to water samples (Luminescent bacteria test with Biotox testing kit; ISO 11348-3, 2009, Environmental Bio-Detection Products Inc, Campobello, Ontario, Canada). The test involves the standard procedure for Microtox analysis [
57]. Adjusting the osmotic pressure close to 2% NaCl and pH between 6 and 8, the test was carried out at 15 °C. To the test solution, 5- and 15-min exposures were measured for bioluminescence inhibition assay. The percentage inhibition of the luminescence relative to a non-contaminated blank (ultrapure water) was used to express the toxicity of each sample. The EC50 was calculated and subsequently converted into toxicity units (TUs), when the relative inhibition percentage was found to be above 20%. Moreover, the toxicity data from Microtox software
® indicated that for the two contact times (5 and 15 min), there was no statistically significant difference (
p < 0.01); so, the toxicity data at 5 min was considered in this study.
3.7. Identification of NAP Transformation Byproducts
An aliquot of 6 mL of the treated NAP effluent was analyzed by a dispersive liquid–liquid micro extraction procedure [
58]. Then, the sample was injected for analysis in an ion trap mass spectrometer (HCTultra PTM Discovery System, Bruker, Billerica, MA. USA) coupled to HPLC with ESI, APCI, and NS interfaces (mass range: 50–6000 uma). The turbo ion spray source was operated in negative ion mode for all the analytes.
4. Conclusions
The effect of the operation parameters, e.g., temperature, H2O2 dosage, and pH, on the degradation of naproxen by catalytic wet peroxide oxidation (CWPO) using a laboratory-synthesized magnetic Fe3O4/MWCNTs catalyst was evaluated. The optimization of the experimental study was assessed using a response surface methodology (RSM)-Box–Behnken Design (BBD). The results showed that a high removal efficiency of the pollutant (82%) was obtained at 70 °C of temperature, 1.5 mM of H2O2 dose. and a pH value of 5. Furthermore, the performance of the catalyst was favored at low pH values and high temperatures. Indeed, it was found that hydrogen peroxide dosages higher than 1.5 mM decreased the removal of naproxen. The reaction kinetics were shown to be adequately described by the pseudo-second-order model, finding an activation energy value of 4.75 KJ/mol. Related to its stability, the catalyst showed a high removal efficiency (~80%) without loss of activity along three consecutive runs.
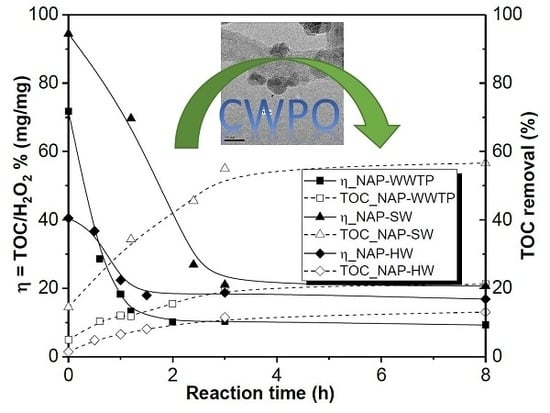
Two possible naproxen reaction mechanisms based on •OH radicals attack have been proposed. The degradation of several real-aqueous matrices—a wastewater treatment plant effluent (WWTP), surface water (SW), and a hospital wastewater (HW) spiked with naproxen—was evaluated, finding removal percentages of TOC of 50%, 15%, and 10% for NAP-SW, NAP-HW, and NAP-WWTP, respectively. Low efficiencies can be attributed to the high content of aromatic and nitrogenated compounds of the samples that act as scavengers of H2O2. Finally, the toxicity of the treated real effluents was measured, obtaining successful results, with a maximum toxicity removal value of 96.5% for the hospital wastewater sample.
So, the obtained results support that the synthesized catalyst can be efficiently used in CWPO processes, revealing a promising and sustainable alternative for the treatment of wastewaters containing highly recalcitrant and toxic micro-pollutants.