Cyclohexene Oxidation with H2O2 over Metal-Organic Framework MIL-125(Ti): The Effect of Protons on Reactivity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalysts Synthesis and Characterization
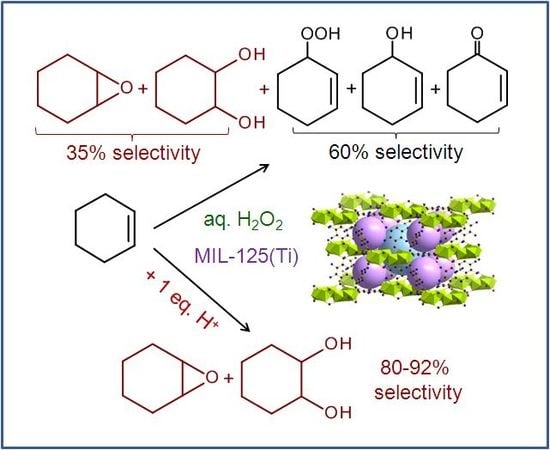
2.2. Catalytic Studies
2.3. Catalyst Recyclability and Stability
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation and Characterization
3.3. Catalytic Oxidations
3.4. Instrumentation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; van Vliet, M.C.A. Epoxidation. In Fine Chemicals through Heterogeneous Catalysis, 1st ed.; Sheldon, R.A., van Bekkum, H., Eds.; Wiley: Weinheim, Germany, 2001; pp. 473–490. [Google Scholar]
- Sienel, G.; Rieth, R.; Rowbottom, K.T. Epoxides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000. [Google Scholar] [CrossRef]
- Dusi, M.; Mallat, T.; Baiker, A. Epoxidation of functionalized olefins over solid catalysts. Catal. Rev. Sci. Eng. 2000, 42, 213–278. [Google Scholar] [CrossRef]
- Catalytic Oxidations with Hydrogen Peroxide as Oxidant; Strukul, G. (Ed.) Kluwer Academic: Dordrecht, The Netherlands, 1992. [Google Scholar]
- Jones, C.W. Application of Hydrogen Peroxide and Derivatives; Royal Society of Chemistry: Cambridge, UK, 1999. [Google Scholar]
- Strukul, G.; Scarso, A. Environmentally benign oxidants. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 1–20. [Google Scholar]
- Notari, B. Microporous crystalline titanium silicates. Adv. Catal. 1996, 41, 253–334. [Google Scholar]
- Clerici, M.G.; Domine, M.E. Oxidation reactions catalyzed by transitionmetal-substituted zeolites. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 21–93. [Google Scholar]
- Buzzoni, R.; Ricci, M.; Rossini, S.; Perego, C. Selective oxidations at Eni. In Handbook of Advanced Methods and Processes in Oxidation Catalysis; Duprez, D., Cavani, F., Eds.; Imperial College Press: London, UK, 2014; pp. 353–381. [Google Scholar]
- Functional Metal-Organic Frameworks: Gas Storage, Separation and Catalysis; Schröder, M. (Ed.) Springer: Heidelberg, Germany, 2010. [Google Scholar]
- Metal-Organic Frameworks: Applications in Separations and Catalysis; García, H.; Navalón, S. (Eds.) Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018. [Google Scholar]
- Elaboration and Applications of Metal-Organic Frameworks, Series on Chemistry, Energy and the Environment; Ma, S.; Perman, J.A. (Eds.) World Scientific Publishing Co. Pte. Ltd.: Singapore, 2018; Volume 2. [Google Scholar]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1201–1508. [Google Scholar] [CrossRef]
- Special Issue on Metal Organic Frameworks. Micropor. Mesopor. Mater. 2012, 157, 1–146. [CrossRef]
- Themed Issue on Metal-Organic Frameworks. Chem. Soc. Rev. 2014, 43, 5415–6172. [CrossRef] [PubMed]
- Special Issue on Chemistry and Applications of Metal Organic Frameworks. Coord. Chem Rev. 2016, 307, 105–424.
- Cluster Issue “Metal–Organic Frameworks Heading towards Application”. Eur. J. Inorg. Chem. 2016, 4265–4529. [CrossRef]
- Special Issue on Coordination Polymers/MOFs. ChemPlusChem 2016, 81, 666–898.
- Cui, Y.; Li, B.; He, H.; Zhou, W.; Chen, B.; Qian, G. Metal–organic frameworks as platforms for functional materials. Acc. Chem. Res. 2016, 49, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Maurin, G.; Serre, C.; Cooper, A.; Férey, G. Metal-Organic Frameworks and Porous Polymers–Current and Future Challenges. special issue on MOFs. Chem. Soc. Rev. 2017, 46, 3104–3107. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Férey, G.; Lee, U.-H.; Chang, J.-S. Liquid phase oxidation of organic compounds by metal-organic frameworks. In Liquid Phase Oxidation via Heterogeneous Catalysis: Organic Synthesis and Industrial Applications; Clerici, M.G., Kholdeeva, O.A., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 371–409. [Google Scholar]
- Luz, I.; León, A.; Boronat, M.; Llabres, X.; Corma, A. Selective aerobic oxidation of activated alkanes with MOFs and their use for epoxidation of olefins with oxygen in a tandem reaction. Catal. Sci. Technol. 2013, 3, 371–379. [Google Scholar] [CrossRef] [Green Version]
- Leus, K.; Liu, Y.-Y.; Van Der Voort, P. Metal-organic frameworks as selective or chiral oxidation catalysts. Catal Rev. 2014, 56, 1–56. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.-Y.; Park, J.; Raiff, A.; Wei, Z.; Zhou, H.-C. Metal–organic frameworks as biomimetic catalysts. ChemCatChem 2014, 6, 67–75. [Google Scholar] [CrossRef]
- Kathalikkattil, A.C.; Babu, R.; Tharun, J.; Roshan, R.; Park, D.-W. Advancements in the conversion of carbon dioxide to cyclic carbonates using metal organic frameworks as catalysts. Catal. Surv. Asia 2015, 19, 223–235. [Google Scholar] [CrossRef]
- Opanasenko, M. Catalytic behavior of metal-organic frameworks and zeolites: Rationalization and comparative analysis. Catal. Today 2015, 243, 2–9. [Google Scholar] [CrossRef]
- Chughtai, A.H.; Ahmad, N.; Younus, H.A.; Laypkov, A.; Verpoort, F. Metal–organic frameworks: Versatile heterogeneous catalysts for efficient catalytic organic transformations. Chem. Soc. Rev. 2015, 44, 6804–6849. [Google Scholar] [CrossRef]
- Kholdeeva, O.A. Liquid-phase selective oxidation catalysis with metal-organic frameworks. Catal Today 2016, 278, 22–29. [Google Scholar] [CrossRef]
- Panchenko, V.N.; Timofeeva, M.N.; Jhung, S.H. Acid-base properties and catalytic activity of metal-organic frameworks: A view from spectroscopic and semiempirical methods. Catal. Rev. 2016, 58, 209–307. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; García, H. Metal–organic frameworks as catalysts for oxidation reactions. Chem. Eur. J. 2016, 22, 8012–8024. [Google Scholar] [CrossRef]
- He, H.; Perman, J.A.; Zhu, G.; Ma, S. Metal-organic frameworks for CO2 chemical transformations. Small 2016, 12, 6309–6324. [Google Scholar] [CrossRef] [PubMed]
- Rogge, S.M.J.; Bavykina, A.; Hajek, J.; Garcia, H.; Olivos-Suarez, A.I.; Sepurlveda-Escribano, A.; Vimont, A.; Clet, G.; Bazin, P.; Kapteijn, F.; et al. Metal–organic and covalent organic frameworks as single-site catalysts. Chem. Soc. Rev. 2017, 46, 3134–3184. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.; Zhao, D. Metal–organic frameworks with Lewis acidity: Synthesis, characterization, and catalytic applications. CrystEngComm 2017, 19, 4066–4081. [Google Scholar] [CrossRef]
- Liang, J.; Liang, Z.; Zou, R.; Zhao, Y. Heterogeneous catalysis in zeolites, mesoporous silica, and metal–organic frameworks. Adv. Mater. 2017, 29, 1701139. [Google Scholar] [CrossRef] [PubMed]
- Dhakshinamoorthy, A.; Asiric, A.M.; Herance, J.R.; García, H. Metal organic frameworks as solid promoters for aerobic autoxidations. Catal. Today 2018, 306, 2–8. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Li, Z.; García, H. Catalysis and photocatalysis by metal organic frameworks. Chem. Soc. Rev. 2018, 47, 8134–8172. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Safarifard, V.; Doustkhah, E.; Rostamnia, S.; Morsali, A.; Nouruzi, N.; Beheshti, S.; Akhbari, K. Taking organic reactions over metal-organic frameworks as heterogeneous catalysis. Micropor. Mesopor. Mater. 2018, 256, 111–127. [Google Scholar] [CrossRef]
- Qin, J.-S.; Yuan, S.; Lollar, C.; Pang, J.; Alsalme, A.; Zhou, H.-C. Stable metal–organic frameworks as a host platform for catalysis and biomimetics. Chem. Commun. 2018, 54, 4231–4249. [Google Scholar] [CrossRef] [PubMed]
- Kramer, S.; Bennedsen, N.R.; Kegnæs, S. Porous organic polymers containing active metal centers as catalysts for synthetic organic chemistry. ACS Catal. 2018, 8, 6961–6982. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Asiri, A.M.; Alvaro, M.; García, H. Metal organic frameworks as catalysts in solvent-free or ionic liquid assisted conditions. Green Chem. 2018, 20, 86–107. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Zalomaeva, O.V.; Skobelev, I.Y.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Metal-organic frameworks of the MIL-101 family as heterogeneous single-site catalysts. Proc. R. Soc. A 2012, 468, 2017–2034. [Google Scholar] [CrossRef]
- Devic, T.; Serre, C. High valence 3p and transition metal based MOFs. Chem. Soc. Rev. 2014, 43, 6097–6115. [Google Scholar] [CrossRef] [PubMed]
- Amador, R.N.; Carboni, M.; Meyer, D. Photosensitive titanium and zirconium metal organic frameworks: Current research and future possibilities. Mater. Lett. 2016, 166, 327–338. [Google Scholar] [CrossRef]
- Sun, D.; Li, Z. Robust Ti-and Zr-based metal-organic frameworks for photocatalysis. Chin. J. Chem. 2017, 35, 135–147. [Google Scholar] [CrossRef]
- Assi, H.; Mouchaham, G.; Steunou, N.; Devic, T.; Serre, C. Titanium coordination compounds: From discrete metal complexes to metal–organic frameworks. Chem. Soc. Rev. 2017, 46, 3431–3452. [Google Scholar] [CrossRef]
- Zhu, J.; Li, P.-Z.; Guo, W.; Zhao, Y.; Zou, R. Titanium-based metal–organic frameworks for photocatalytic applications. Coord. Chem. Rev. 2018, 359, 80–101. [Google Scholar]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Ferey, G. A new photoactive crystalline highly porous titanium (IV) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Zlotea, C.; Phanon, D.; Mazaj, M.; Heurtaux, D.; Guillerm, V.; Serre, C.; Horcajada, P.; Devic, T.; Magnier, E.; Cuevas, F.; et al. Effect of NH2 and CF3 functionalization on the hydrogen sorption properties of MOFs. Dalton Trans. 2011, 40, 4879–4881. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-light-promoted photocatalytic hydrogen production by using an amino-functionalized Ti (IV) metal–organic framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal–organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. 2012, 124, 3364–3367. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Ye, L.; Li, Z. Visible-light-assisted aerobic photocatalytic oxidation of amines to imines over NH2-MIL-125 (Ti). Appl. Catal. B Environm. 2015, 164, 428–432. [Google Scholar] [CrossRef]
- Santaclara, J.G.; Nasalevich, M.A.; Castellanos, S.; Evers, W.H.; Spoor, F.C.M.; Rock, K.; Siebbeles, L.D.A.; Kapteijn, F.; Grozema, F.; Houtepen, A.; et al. Organic linker defines the excited-state decay of photocatalytic MIL-125 (Ti)-type materials. ChemSusChem 2016, 9, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.B.; Wang, X.; Ellezam, L.; Ersen, O.; Fontecave, M.; Sanchez, C.; Rozes, L.; Mellot-Draznieks, C. Maximizing the photocatalytic activity of metal–organic frameworks with aminated-functionalized linkers: Substoichiometric effects in MIL-125-NH2. J. Am. Chem. Soc. 2017, 139, 8222–8228. [Google Scholar] [CrossRef] [PubMed]
- Kampouri, S.; Nguyen, T.N.; Spodaryk, M.; Palgrave, R.G.; Züttel, A.; Smit, B.; Stylianou, K.C. Concurrent photocatalytic hydrogen generation and dye degradation using MIL-125-NH2 under visible light irradiation. Adv. Funct. Mater. 2018, 1806368. [Google Scholar] [CrossRef]
- Wu, Z.; Huang, X.; Zheng, H.; Wang, P.; Hai, G.; Dong, W.; Wang, G. Aromatic heterocycle-grafted NH2-MIL-125 (Ti) via conjugated linker with enhanced photocatalytic activity for selective oxidation of alcohols under visible light. Appl. Catal. B Environm. 2018, 224, 479–487. [Google Scholar] [CrossRef]
- Kim, S.-N.; Kim, J.; Kim, H.-Y.; Cho, H.-Y.; Ahn, W.-S. Adsorption/catalytic properties of MIL-125 and NH2-MIL-125. Catal. Today 2013, 204, 85–93. [Google Scholar] [CrossRef]
- McNamara, N.D.; Neumann, G.T.; Masko, E.T.; Urban, J.A.; Hicks, J.C. Catalytic performance and stability of (V) MIL-47 and (Ti) MIL-125 in the oxidative desulfurization of heterocyclic aromatic sulfur compounds. J. Catal. 2013, 305, 217–226. [Google Scholar] [CrossRef]
- Ivanchikova, I.D.; Lee, J.S.; Maksimchuk, N.V.; Shmakov, A.N.; Chesalov, Y.A.; Ayupov, A.B.; Hwang, Y.K.; Jun, C.-H.; Chang, J.-S.; Kholdeeva, O.A. Highly selective H2O2-based oxidation of alkylphenols to p-benzoquinones over MIL-125 metal–organic frameworks. Eur. J. Inorg. Chem. 2014, 132–139. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Ivanchikova, I.D.; Maksimchuk, N.V.; Mel’gunov, M.S.; Chang, J.-S.; Guidotti, M.; Shutilov, A.A.; Zaikovskii, V.I. Environmentally benign oxidation of alkylphenols to p-benzoquinones: A comparative study of various Ti-containing catalysts. Top. Catal. 2014, 57, 1377–1384. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Song, J.Y.; Khan, N.A.; Jhung, S.H. TiO2-containing carbon derived from a metal–organic framework composite: A highly active catalyst for oxidative desulfurization. ACS Appl. Mater. Interfaces 2017, 9, 31192–31202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lia, G.; Kong, L.; Lu, H. Deep oxidative desulfurization catalyzed by Ti-based metal-organic frameworks. Fuel 2018, 219, 103–110. [Google Scholar] [CrossRef]
- Vermoortele, F.; Maes, M.; Moghadam, P.Z.; Lennox, M.J.; Ragon, F.; Boulhout, M.; Biswas, S.; Laurier, K.G.M.; Beurroies, I.; Denoyel, R.; et al. p-Xylene-selective metal–organic frameworks: A case of topology-directed selectivity. J. Am. Chem. Soc. 2011, 133, 18526–18529. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Kochi, J.K. Metal–Catalyzed Oxidations of Organic Compounds; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Kholdeeva, O.A.; Maksimov, G.M.; Maksimovskaya, R.I.; Kovaleva, L.A.; Fedotov, M.A. Role of protons in methyl phenyl sulfide oxidation with hydrogen peroxide catalyzed by Ti (IV)-monosubstituted heteropolytungstates. React. Kinet. Catal. Lett. 1999, 66, 311–317. [Google Scholar] [CrossRef]
- Kamata, K.; Yonehara, K.; Sumida, Y.; Yamaguchi, K.; Hikichi, S.; Mizuno, N. Efficient epoxidation of olefins with ≥99% selectivity and use of hydrogen peroxide. Science 2003, 300, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Kholdeeva, O.A.; Trubitsina, T.A.; Timofeeva, M.N.; Maksimov, G.M.; Maksimovskaya, R.I.; Rogov, V.A. The role of protons in cyclohexene oxidation with H2O2 catalysed by Ti (IV)-monosubstituted Keggin polyoxometalate. J. Mol. Catal. A Chem. 2005, 232, 173–178. [Google Scholar] [CrossRef]
- Kholdeeva, O.A.; Maksimovskaya, R.I. Titanium- and zirconium-monosubstituted polyoxometalates as molecular models for studying mechanisms of oxidation catalysis. J. Mol. Catal. A Chem. 2007, 262, 7–24. [Google Scholar] [CrossRef]
- Sartorel, A.; Carraro, M.; Bagno, A.; Scorrano, G.; Bonchio, M. Asymmetric tetraprotonation of γ-[(SiO4)W10O32]8− triggers a catalytic epoxidation reaction: Perspectives in the assignment of the active catalyst. Angew. Chem. Int. Ed. 2007, 46, 3255–3258. [Google Scholar] [CrossRef]
- Mizuno, N.; Kamata, K.; Yamaguchi, K. Oxidative functional group transformations with hydrogen peroxide catalyzed by a divanadium-substituted phosphotungstate. Catal. Today 2012, 185, 157–161. [Google Scholar] [CrossRef]
- Jiménez-Lozano, P.; Ivanchikova, I.D.; Kholdeeva, O.A.; Poblet, J.M.; Carbó, J.J. Alkene oxidation by Ti-containing polyoxometalates. Unambiguous characterization of the role of the protonation state. Chem. Commun. 2012, 48, 9266–9268. [Google Scholar] [CrossRef] [PubMed]
- Kholdeeva, O.A. Hydrogen peroxide activation over TiIV: What have we learned from studies on Ti-containing polyoxometalates? Eur. J. Inorg. Chem. 2013, 1595–1605. [Google Scholar] [CrossRef]
- Satake, N.; Hirano, T.; Kamata, K.; Suzuki, K.; Yamaguchi, K.; Mizuno, N. Synthesis, structural characterization, and oxidation catalysis of a diniobium-substituted silicodecatungstate. Chem. Lett. 2015, 44, 899–901. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Maksimov, G.M.; Evtushok, V.Yu.; Ivanchikova, I.D.; Chesalov, Yu.A.; Maksimovskaya, R.I.; Kholdeeva, O.A.; Solé-Daura, A.; Poblet, J.M.; Carbó, J.J. Relevance of protons in heterolytic activation of H2O2 over Nb(V): Insights from model studies on nb-substituted polyoxometalates. ACS Catal. 2018, 8, 9722–9737. [Google Scholar] [CrossRef]
- Yoon, C.W.; Hirsekorn, K.F.; Neidig, M.L.; Yang, X.; Tilley, T.D. Mechanism of the decomposition of aqueous hydrogen peroxide over heterogeneous TiSBA15 and TS-1 selective oxidation catalysts: Insights from spectroscopic and density functional theory studies. ACS Catal. 2011, 1, 1665–1678. [Google Scholar] [CrossRef]
- Bordiga, S.; Groppo, E.; Agostini, G.; van Bokhoven, J.A.; Lamberti, C. Reactivity of surface species in heterogeneous catalysts probed by in situ X-ray absorption techniques. Chem. Rev. 2013, 113, 1736–1850. [Google Scholar] [CrossRef] [PubMed]
- Kholdeeva, O.A.; Ivanchikova, I.D.; Maksimchuk, N.V.; Skobelev, I.Y. H2O2-based selective epoxidations: Nb-silicates versus Ti-silicates. Catal. Today 2018. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Wallau, M.; Arends, I.W.C.E.; Schuchardt, U. Heterogeneous catalysts for liquid-phase oxidations: Philosophers’ stones or Trojan horses? Acc. Chem. Res. 1998, 31, 485–493. [Google Scholar] [CrossRef]
- Llabres, X.; Casanova, O.; Galiasso Tailleur, R.; Garcia, H.; Corma, A. Metal organic frameworks (MOFs) as catalysts: A combination of Cu2+ and Co2+ MOFs as an efficient catalyst for tetralin oxidation. J. Catal. 2008, 255, 220–227. [Google Scholar] [CrossRef]
- Torbina, V.V.; Ivanchikova, I.D.; Kholdeeva, O.A.; Skobelev, I.Y.; Vodyankina, O.V. Propylene glycol oxidation with tert-butyl hydroperoxide over Cr-containing metal-organic frameworks MIL-101 and MIL-100. Catal. Today 2016, 278, 97–103. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A 2002, 189, 39–66. [Google Scholar] [CrossRef]








| Entry | MOF | Particle Size, µm | SBET, m2/g | Vp, a cm3/g |
|---|---|---|---|---|
| 1 | MIL-125-S | 0.5 | 1591 | 0.59 |
| 2 | MIL-125-M | 1.5 | 1537 | 0.58 |
| 3 | MIL-125-L | 5.0 | 1511 | 0.57 |
| 4 | 1373 b | 0.47 b |
| Entry | Catalyst | T, °C | Time, min | CyH Conv. b, % | Product Selectivity c, % | ||
|---|---|---|---|---|---|---|---|
| Epoxide | Diol | Allylic d | |||||
| 1 | – e | 50 | 60 | 6 | 16 | 16 | 65 |
| 2 | H+ f | 50 | 60 | 8 | 25 | 26 | 47 |
| 3 | MIL-125-M | 50 | 60 | 26 | 25 | 10 | 60 |
| 4 | MIL-125-M + 1 eq. H+ | 50 | 45 | 42 | 48 | 27 | 22 |
| 5 | MIL-125-M + 1 eq. H+ g | 50 | 45 | 43 | 17 | 63 | 19 |
| 6 | MIL-125-M + 1 eq. H+ | 30 | 90 | 24 | 63 | 9 | 26 |
| 7 | MIL-125-S + 0.5 eq. H+ h | 50 | 50 | 30 | 35 | 36 | 28 |
| 8 | MIL-125-S + 1 eq. H+ | 50 | 45 | 38 | 42 | 34 | 22 |
| 9 | MIL-125-S + 1.5 eq. H+ i | 50 | 30 | 41 | 45 | 43 | 9 |
| 10 | MIL-125-L + 1 eq. H+ | 50 | 45 | 41 | 40 | 38 | 21 |
| Entry | Catalyst | Oxidant | Time, min | CyH Conv. b, % | Product Selectivity c, % | ||
|---|---|---|---|---|---|---|---|
| Epoxide | Diol | Allylic d | |||||
| 1 | MIL-125-M | tBuOOH | 60 | 25 | 16 | 28 | 54 |
| 2 | MIL-125-M + 1 eq. H+ e | tBuOOH | 45 | 46 | 13 | 17 f | 45 |
| 3 | MIL-125-S + 1 eq. H+ | 30% H2O2 | 45 | 38 | 43 | 33 | 22 |
| 4 | MIL-125-S + 1 eq. H+ | 50% H2O2 | 45 | 40 | 57 | 35 | 6 |
| 5 | MIL-125-L + 1 eq. H+ | 30% H2O2 | 45 | 41 | 40 | 38 | 21 |
| 6 | MIL-125-L + 1 eq. H+ | 50% H2O2 | 45 | 41 | 45 | 47 | 7 |
| Run | CyH Conv., % | Selectivity, % | ||
|---|---|---|---|---|
| Epoxide | Diol | Allylic b | ||
| 1 | 41 | 40 | 38 | 21 |
| 2 | 39 | 42 | 32 | 23 |
| 3 | 39 | 48 | 28 | 21 |
| 4 | 40 | 54 | 29 | 14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimchuk, N.; Lee, J.S.; Ayupov, A.; Chang, J.-S.; Kholdeeva, O. Cyclohexene Oxidation with H2O2 over Metal-Organic Framework MIL-125(Ti): The Effect of Protons on Reactivity. Catalysts 2019, 9, 324. https://doi.org/10.3390/catal9040324
Maksimchuk N, Lee JS, Ayupov A, Chang J-S, Kholdeeva O. Cyclohexene Oxidation with H2O2 over Metal-Organic Framework MIL-125(Ti): The Effect of Protons on Reactivity. Catalysts. 2019; 9(4):324. https://doi.org/10.3390/catal9040324
Chicago/Turabian StyleMaksimchuk, Nataliya, Ji Sun Lee, Artem Ayupov, Jong-San Chang, and Oxana Kholdeeva. 2019. "Cyclohexene Oxidation with H2O2 over Metal-Organic Framework MIL-125(Ti): The Effect of Protons on Reactivity" Catalysts 9, no. 4: 324. https://doi.org/10.3390/catal9040324
APA StyleMaksimchuk, N., Lee, J. S., Ayupov, A., Chang, J. -S., & Kholdeeva, O. (2019). Cyclohexene Oxidation with H2O2 over Metal-Organic Framework MIL-125(Ti): The Effect of Protons on Reactivity. Catalysts, 9(4), 324. https://doi.org/10.3390/catal9040324