Catalytic Behaviour of Ce-Doped Ni Systems Supported on Stabilized Zirconia under Dry Reforming Conditions
Abstract
:1. Introduction
2. Results and Discussion
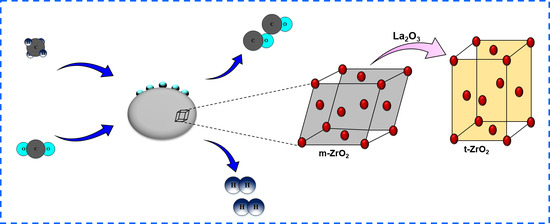
2.1. Physicochemical Features of the Ceria Promoted Ni/x-Zr (x = 0, La2O3) Catalysts
2.2. Catalytic Activity and Stability
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Catalytic Testing
3.3. Catalyst Characterization
3.3.1. XRD Characterization
3.3.2. N2 Physisorption
3.3.3. Temperature Programmed Reduction (TPR)
3.3.4. H2 Temperature-Programmed Desorption
3.3.5. CO2 Temperature-Programmed Desorption (CO2-TPD)
3.3.6. Thermo-Gravimetric Analysis (TGA)
3.3.7. TEM Characterization
3.3.8. XPS Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pan, X.; Fan, Z.; Chen, W.; Ding, Y.; Luo, H.; Bao, X. Enhanced ethanol production inside carbon-nanotube reactors containing catalytic particles. Nat. Mat. 2007, 6, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Hamza Fakeeha, A.; Arafat, Y.; Aidid Ibrahim, A.; Shaikh, H.; Atia, H.; Elhag Abasaeed, A.; Armbruster, U.; Sadeq Al-Fatesh, A. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni–Co)/ZrO2–Al2O3 Catalysts: Influence of Calcination Temperature. Processes 2019, 7, 141. [Google Scholar] [CrossRef]
- Gao, X.Y.; Hidajat, K.; Kawi, S. Facile synthesis of Ni/SiO2 catalyst by sequential hydrogen/air treatment: A superior anti-coking catalyst for dry reforming of methane. J. CO2 Util. 2016, 15, 146–153. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Q.; Liu, B.; Jiang, F.; Xu, Y.; Liu, X. A Facile Fabrication of Supported Ni/SiO2 Catalysts for Dry Reforming of Methane with Remarkably Enhanced Catalytic Performance. Catalysts 2019, 9, 183. [Google Scholar] [CrossRef]
- Karam, L.; Casale, S.; El Zakhem, H.; El Hassan, N. Tuning the properties of nickel nanoparticles inside SBA-15 mesopores for enhanced stability in methane reforming. J. CO2 Util. 2017, 17, 119–124. [Google Scholar] [CrossRef]
- El Hassan, N.; Kaydouh, M.; Geagea, H.; El Zein, H.; Jabbour, K.; Casale, S.; El Zakhem, H.; Massiani, P. Low temperature dry reforming of methane on rhodium and cobalt based catalysts: Active phase stabilization by confinement in mesoporous SBA-15. Appl. Catal. A Gen. 2016, 520, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Al-Fatesh, A.S.; Arafat, Y.; Atia, H.; Ibrahim, A.A.; Ha, Q.L.M.; Schneider, M.; M-Pohl, M.; Fakeeha, A.H. CO2-reforming of methane to produce syngas over Co-Ni/SBA-15 catalyst: Effect of support modifiers (Mg, La and Sc) on catalytic stability. J. CO2 Util. 2017, 21, 395–404. [Google Scholar] [CrossRef]
- Moradi, G.R.; Rahmanzadeh, M.; Khosravian, F. The effects of partial substitution of Ni by Zn in LaNiO3 perovskite catalyst form ethane dry reforming. J. CO2 Util. 2014, 6, 7–11. [Google Scholar] [CrossRef]
- Kambolis, A.; Matralis, H.; Trovarelli, A.; Papadopoulou, C. Ni/CeO2-ZrO2 catalysts for the dry reforming of methane. Appl. Catal. A 2010, 377, 16–26. [Google Scholar] [CrossRef]
- Lemonidou, A.A.; Vasalos, I.A. Carbon dioxide reforming of methane over 5 wt.% Ni/CaO-Al2O3 catalyst. Appl. Catal. A 2002, 228, 227–235. [Google Scholar] [CrossRef]
- Han, J.W.; Park, J.S.; Choi, M.S.; Lee, H. Uncoupling the size and support effects of Ni catalysts for dry reforming of methane. Appl. Catal. B 2017, 203, 625–632. [Google Scholar] [CrossRef]
- Świrk, K.; Gálvez, M.E.; Motak, M.; Grzybek, T.; Rønning, M.; Da Costa, P. Dry reforming of methane over Zr- and Y-modified Ni/Mg/Al double-layered hydroxides. Catal. Commun. 2018, 117, 26–32. [Google Scholar]
- Sarkar, D.; Adak, S.; Chu, M.; Cho, S.; Mitra, N. Influence of ZrO2 on the thermo-mechanical response of nano-ZTA. Ceram. Int. 2007, 33, 255–261. [Google Scholar] [CrossRef]
- Lercher, J.; Bitter, J.; Hally, W.; Niessen, W.; Seshan, K. Design of stable catalysts for methane-carbon dioxide reforming. Studies Surf. Sci. Catal. 1996, 101, 463–472. [Google Scholar] [Green Version]
- Li, X.; Chang, J.; Park, S. Carbon as an intermediate during the carbon dioxide reforming of methane over zirconia-supported high nickel loading catalysts. Chem. Lett. 1999, 10, 1099–1100. [Google Scholar] [CrossRef]
- Chuah, G.K.; Liu, S.H.; Jaenicke, S.; Li, J. High surface area zirconia by digestion of zirconium propoxide at different pH. Microporous Mesoporous Mat. 2000, 39, 381–392. [Google Scholar] [CrossRef]
- Jaenicke, S.; Chuah, G.K.; Raju, V.; Nie, Y.T. Structural and Morphological Control in the Preparation of High Surface Area Zirconia. Catal. Surv. Asia 2008, 12, 153–169. [Google Scholar] [CrossRef]
- Fan, M.-S.; Abdullah, A.Z.; Bhatia, S. Utilization of greenhouse gases through carbon dioxide reforming of methane over Ni–Co/MgO–ZrO2: preparation, characterization and activity studies. Appl. Catal. B 2010, 100, 365–377. [Google Scholar] [CrossRef]
- Yamasaki, M.; Habazaki, H.; Asami, K.; Izumiya, K.; Hashimoto, K. Effect of tetragonal ZrO2 on the catalytic activity of Ni/ZrO2 catalyst prepared from amorphous Ni–Zr alloys. Catal. Commun. 2006, 7, 24–28. [Google Scholar] [CrossRef]
- Rezaei, M.; Alavi, S.M.; Sahebdelfar, S.; Yan, Z.-F. A highly stable catalyst in methane reforming with carbon dioxide. Scripta Materialia 2009, 61, 173–176. [Google Scholar] [CrossRef]
- Choque, V.; de la Piscina, P.R.; Molyneux, D.; Homs, N. Ruthenium supported on new TiO2–ZrO2 systems as catalysts for the partial oxidation of methane. Catal. Today 2010, 149, 248–253. [Google Scholar] [CrossRef]
- Verykios, X.E. Catalytic dry reforming of natural gas for the production of chemicals and hydrogen. Int. J. Hydrogen Energy 2003, 28, 1045–1063. [Google Scholar] [CrossRef]
- Slagtern, A.; Schuurman, Y.; Leclercq, C.; Verykios, X.; Mirodatos, C. Specific features concerning the mechanism of methane reforming by carbon dioxide over Ni/La2O3 catalyst. J. Catal. 1997, 172, 118–126. [Google Scholar] [CrossRef]
- Titus, J.; Roussiere, T.; Wasserschaff, G.; Schunk, S.; Milanov, A.; Schwab, E.; Wagner, G.; Oeckler, O.; Gläser, R. Dry reforming of methane with carbon dioxide over NiO–MgO–ZrO2. Catal. Today 2016, 270, 68–75. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl. Catal. B 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; Arandiyan, H.; Peng, Y.; Chang, H.; Li, J. Low temperature complete combustion of methane over cobalt chromium oxides catalysts. Catal. Today 2013, 201, 12–18. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar]
- Rotgerink, H.L.; Paalman, R.; Van Ommen, J.; Ross, J. Studies on the promotion of nickel—alumina coprecipitated catalysts: II. Lanthanum oxide. Appl. Catal. 1988, 45, 257–280. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.; Millar, G.J. Carbon dioxide reforming of methane to produce synthesis gas over metal-supported catalysts: state of the art. Energy Fuels 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Kumar, P.; Sun, Y.; Idem, R.O. Comparative Study of Ni-based Mixed Oxide Catalyst for Carbon Dioxide Reforming of Methane. Energy Fuels 2008, 22, 3575–3582. [Google Scholar] [CrossRef]
- Huang, S.J.; Walters, A.B.; Vannice, M.A. TPD, TPR and DRIFTS studies of adsorption and reduction of NO on La2O3 dispersed on Al2O3. Appl. Catal. B 2000, 26, 101–118. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Biesinger, M.C.; Smart, R.S.C.; McIntyre, N.S. New interpretations of XPS spectra of nickel metal and oxides. Surf.Sci. 2006, 600, 1771–1779. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in depth investigation of deactivation through carbon formation during the biogas dry reforming reaction for Ni supported on modified with CeO2 and La2O3 zirconia catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Liu, G.; Liu, A.; Meng, Y.; Shan, F.; Shin, B.; Lee, W.; Cho, C. Annealing dependence of solution-processed ultra-thin ZrOx films for gate dielectric applications. J. Nanosci. Nanotechnol. 2015, 15, 2185–2191. [Google Scholar] [CrossRef]
- Italiano, G.; Delia, A.; Espro, C.; Bonura, G.; Frusteri, F. Methane decomposition over Co thin layer supported catalysts to produce hydrogen for fuel cell. Int. J. Hydrogen Energy 2010, 35, 11568–11575. [Google Scholar] [CrossRef]
- Frusteri, F.; Italiano, G.; Espro, C.; Cannilla, C.; Bonura, G. H2 production by methane decomposition: Catalytic and technological aspects. Int. J. Hydrogen Energy 2012, 37, 16367–16374. [Google Scholar] [CrossRef]
- Frusteri, F.; Frontera, P.; Modafferi, V.; Bonura, G.; Bottari, M.; Siracusano, S.; Antonucci, P.L. Catalytic features of Ni/Ba–Ce0. 9–Y0. 1 catalyst to produce hydrogen for PCFCs by methane reforming. Int. J. Hydrogen Energy 2010, 35, 11661–11668. [Google Scholar]
- Frontera, P.; Macario, A.; Monforte, G.; Bonura, G.; Ferraro, M.; Dispenza, G.; Antonucci, V.; Aricò, A.S.; Antonucci, P.L. The role of Gadolinia Doped Ceria support on the promotion of CO2methanation over Ni and NiFe catalysts. Int. J. Hydrogen Energy 2017, 42, 26828–26842. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Atia, H.; Fakeeha, A.H.; Armbruster, U.; Abasaeed, A.E.; Frusteri, F. Evaluation of Co-Ni/Sc-SBA–15 as a novel coke resistant catalyst for syngas production via CO2 reforming of methane. Appl. Catal. A 2018, 567, 102–111. [Google Scholar] [CrossRef]
- Bonura, G.; Di Blasi, O.; Spadaro, L.; Arena, F.; Frusteri, F. A basic assessment of the reactivity of Ni catalysts in the decomposition of methane for the production of “COx-free” hydrogen for fuel cells application. Catal. Today 2006, 116, 298–303. [Google Scholar] [CrossRef]
- Frusteri, F.; Spadaro, L.; Arena, F.; Chuvilin, A. TEM evidence for factors affecting the genesis of carbon species on bare and K-promoted Ni/MgO catalysts during the dry reforming of methane. Carbon 2002, 40, 1063–1070. [Google Scholar] [CrossRef]
- Branca, C.; Frusteri, F.; Magazzù, V.; Mangione, A. Characterization of carbon nanotubes by TEM and infrared spectroscopy. J. Phys. Chem. B 2004, 108, 3469–3473. [Google Scholar] [CrossRef]











| SAMPLE | BET (m2/g) | P.V. (cm3/g) | P.D. (nm) | H2 Consumption (µmol/g) |
|---|---|---|---|---|
| La-Zr Ni/Zr | 67 39 | 0.247 0.111 | 4.0 11.3 | - 1049.3 |
| Ni/La-Zr | 62 | 0.246 | 15.8 | 814.2 |
| Ni-Ce/Zr | 41 | 0.139 | 13.3 | 945.5 |
| Ni-Ce/La-Zr | 65 | 0.242 | 15.0 | 782.2 |
| SAMPLE | H2 uptake (µmol/gcat) | MSA a (m2/gcat) | DNib (%) | dNic (nm) |
|---|---|---|---|---|
| Ni/Zr | 107.3 | 8.4 | 25.2 | 4.0 |
| Ni/La-Zr | 253.3 | 19.8 | 59.6 | 1.7 |
| Ni-Ce/Zr | 173.5 | 13.6 | 40.8 | 2.5 |
| Ni-Ce/La-Zr | 182.5 | 14.3 | 42.9 | 2.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fatesh, A.S.; Arafat, Y.; Ibrahim, A.A.; Kasim, S.O.; Alharthi, A.; Fakeeha, A.H.; Abasaeed, A.E.; Bonura, G.; Frusteri, F. Catalytic Behaviour of Ce-Doped Ni Systems Supported on Stabilized Zirconia under Dry Reforming Conditions. Catalysts 2019, 9, 473. https://doi.org/10.3390/catal9050473
Al-Fatesh AS, Arafat Y, Ibrahim AA, Kasim SO, Alharthi A, Fakeeha AH, Abasaeed AE, Bonura G, Frusteri F. Catalytic Behaviour of Ce-Doped Ni Systems Supported on Stabilized Zirconia under Dry Reforming Conditions. Catalysts. 2019; 9(5):473. https://doi.org/10.3390/catal9050473
Chicago/Turabian StyleAl-Fatesh, Ahmed Sadeq, Yasir Arafat, Ahmed Aidid Ibrahim, Samsudeen Olajide Kasim, Abdulrahman Alharthi, Anis Hamza Fakeeha, Ahmed Elhag Abasaeed, Giuseppe Bonura, and Francesco Frusteri. 2019. "Catalytic Behaviour of Ce-Doped Ni Systems Supported on Stabilized Zirconia under Dry Reforming Conditions" Catalysts 9, no. 5: 473. https://doi.org/10.3390/catal9050473
APA StyleAl-Fatesh, A. S., Arafat, Y., Ibrahim, A. A., Kasim, S. O., Alharthi, A., Fakeeha, A. H., Abasaeed, A. E., Bonura, G., & Frusteri, F. (2019). Catalytic Behaviour of Ce-Doped Ni Systems Supported on Stabilized Zirconia under Dry Reforming Conditions. Catalysts, 9(5), 473. https://doi.org/10.3390/catal9050473