Flexibility in Metal–Organic Frameworks: A Basic Understanding
Abstract
:1. Introduction about Metal–Organic Frameworks (MOFs) and Their Structure
1.1. Origin of MOFs Flexibility
1.2. The Flexibility of Linkers/Functionalized Linkers
- Aromatic rings with the capability of rotation or movement of dangling side chains of the organic ligands [20].
- Ligands with an open structure. This type of ligand depends on the formation of coordination bonds with the central metal atom M. The decrease of the dentation gives multiple degrees of rotational freedom of the ligand around the inorganic moiety. The ratio of the metal/linker (M/L) and linker/linker (L/L) and the synergistic effect of metal nodes and organic linker on framework flexibility are major-league factors that play a vital role in determining the degree of flexibility [21,22].
- Linkers’ surfaces can also be used as anchoring points for introducing additional functionalities to tailor the framework flexibility by the substituent effects at the linker [23]. Such functionalization results in multivariate MOFs (MTV-MOFs).
1.2.1. Carboxylate Linkers and Their Isomerism Features
1.2.2. Mixed-Linker
1.3. Flexibility of the Metal Nodes
2. General Aspects of Framework Flexibility
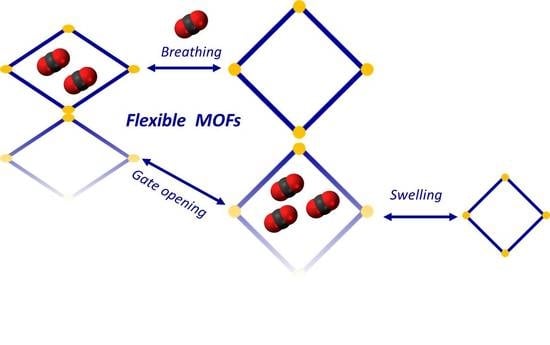
2.1. Breathing, Swelling, and Linker Rotation
2.1.1. Breathing
- The dynamic behavior of the inorganic building units (IBU) e.g., Figure 3 (I), is subject to the ordered rotation of the ligand molecules or a hinge motion of the linkers [28,73,74]. These dynamic behaviors result in opening or closing of pores and accordingly affect the loss/uptake of guest molecules. Carboxylate ligands are considered an example for weak IBU since they are capable of switching their binding mode in the so-called kneecap mechanism in which the ligand rotates around the O–O-axis of the carboxylate group.
- When organic linkers with reversible structural and low energy are considered, one must keep in mind that guests’ exposure will lead to many isomerized linkers; see Figure 3 (II). The reversible photoisomerization of azobenzene molecules with alternating UV/visible light irradiation is a good example of such type of flexible transformation [75].
2.1.2. Swelling
2.1.3. Linker Rotation
2.2. Thermo-Responsivity
2.3. Mechanical Properties, Elasticity
2.4. Photo-Responsiveness
3. How to Control MOFs Flexibility
3.1. Ligand Control
3.1.1. Linker Substitution
3.1.2. Linker Rotation
3.1.3. Post-Synthetic Modification (PSM)
3.2. Metal Ion Modification
4. Characterization Techniques to Detect Flexibility
4.1. Nuclear Magnetic Resonance (NMR)
4.2. Powder X-ray Diffraction (PXRD)
4.3. Infrared Spectroscopy (IR)
4.4. Raman Spectroscopy
5. Application
5.1. Catalytic Application
5.2. Separation
5.3. Guest Capture
5.4. Sensing
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTC | Azobenzenetetra carboxylate |
| BDC | Benzene dicarboxylate |
| BDP | 1,4-benzene dipyrazolate |
| BHE-bpb | 2,5-bis(2-hydroxyethoxy)-1,4-bis(4-pyridyl)benzene |
| bmb | 1,4-bis(2-methylbenzimidazol-1-ylmethyl) benzene |
| BPE | Bis-pyridyl ethylene |
| BTC | Benzene tricarboxylate |
| CP | Closed Pores |
| SCC | Chalcogenolate cluster |
| FL-MOFs | Flexible ligand metal organic framework |
| H3L | 5-(2-carboxybenzyloxy) |
| HKUST-1 | Hong Kong University of Science and Technology |
| IBU | Inorganic building unit |
| IR | Infrared spectroscopy |
| JLU-Liu4 | JiLin University |
| L | Linker |
| LP | Large Pores |
| M | Metal |
| MIL | Materials of Institute Lavoisier |
| MTV | multivariable |
| NMR | Nuclear Magnetic Resonance |
| NP | Narrow pores |
| PCP | Porous coordination polymers |
| PDC | Pyridine dicarboxylic acid |
| PSM | Post-synthetic modifications |
| PXRD | Powder X-ray diffraction |
| Pz | pyrazolate |
| Pzdc | 2,3-pyrazinedicarboxylate |
| SALI | Solvent assisted ligand incorporation |
| SBBs | Supramolecular building blocks |
| SBUs | Secondary building units |
| TM | Transition metal |
| VOC | Volatile Organic Compound |
| ZIF | Zeolitic Imidazolate Framework |
| ZIF-8 | [Zn(mIm)2]n (mIm, also Im) = 2-methylimidazole |
References
- Kinoshita, Y.; Matsubara, I.; Saito, Y. The crystal ntructure of Bis(succinonitrilo) copper (I) nitrate. Bull. Chem. Soc. Jpn. 1959, 32, 741–747. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Matsubara, I.; Saito, Y. The crystal structure of Bis ( glutaronitrilo) copper (I) nitrate. Bull. Chem. Soc. Jpn. 1959, 32, 1216–1221. [Google Scholar] [CrossRef]
- Berlin, A.A.; Matveeva, N.G. Polymeric chelate compounds. Russ. Chem. Rev. 1960, 29, 119–128. [Google Scholar] [CrossRef]
- Block, B.P.; Rose, S.H.; Schaumann, C.W.; Roth, E.S.; Simkin, J. Coordination polymers with inorganic backbones formed by double-bridging of tetrahedral elements. J. Am. Chem. Soc. 1962, 84, 3200–3201. [Google Scholar] [CrossRef]
- Hoskins, B.F.; Robson, R. Design and construction of a new class of scaffolding-like materials comprising infinite polymeric frameworks of 3D-linked molecular rods. A reappraisal of the Zn(CN)2 and Cd(CN)2 structures and the synthesis and structure of the dia. J. Am. Chem. Soc. 1990, 112, 1546–1554. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kondo, M. Functional micropore chemistry of crystalline metal complex-assembled compounds. Bull. Chem. Soc. Jpn. 1998, 71, 1739. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H. Hydrothermal synthesis of a metal-organic framework containing large rectangular channels. J. Am. Chem. Soc. 1995, 117, 10401–10402. [Google Scholar] [CrossRef]
- Riou, D.; Fe, G. Oxidation state of vanadium within the same structural type. J. Mater. Chem. 1998, 8, 2733–2735. [Google Scholar] [CrossRef]
- O’keeffe, M.; Peskov, M.A.; Ramsden, S.J.; Yaghi, O.M. The reticular chemistry structure resource (RCSR) database of, and symbols for, crystal nets. Acc. Chem. Res. 2008, 41, 1782–1789. [Google Scholar] [CrossRef]
- Tranchemontagne, D.J.; Ni, Z.; O’Keeffe, M.; Yaghi, O.M. Reticular chemistry of metal-organic polyhedra. Angew. Chem. Int. Ed. 2008, 47, 5136–5147. [Google Scholar] [CrossRef]
- Perry VI, J.J.; Perman, J.A.; Zaworotko, M.J. Design and synthesis of metal-organic frameworks using metal-organic polyhedra as supermolecular building blocks. Chem. Soc. Rev. 2009, 38, 1400–1417. [Google Scholar] [CrossRef] [PubMed]
- Tranchemontagne, D.J.; Mendoza-Cortés, J.L.; O’Keeffe, M.; Yaghi, O.M. Secondary building units, nets and bonding in the chemistry of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1257–1283. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-J.; Lü, J.; Hong, M.; Cao, R. Metal-organic frameworks based on flexible ligands (FL- MOFs): Structures and applications. Chem. Soc. Rev. 2014, 43, 5867–5895. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wei, Z.; Gu, Z.Y.; Liu, T.F.; Park, J.; Park, J.; Tian, J.; Zhang, M.; Zhang, Q.; Gentle, T.; et al. Tuning the structure and function of metal-organic frameworks via linker design. Chem. Soc. Rev. 2014, 43, 5561–5593. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the crystal structures of metal organic frameworks. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Stock, N.; Biswas, S. Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites. Chem. Rev. 2012, 933–969. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Lee, K.J.; Choi, M.; Park, N.; Moon, D.; Moon, H.R. Metal-organic frameworks constructed from flexible ditopic ligands: Conformational diversity of an aliphatic ligand. New J. Chem. 2013, 37, 4130–4139. [Google Scholar] [CrossRef]
- Wang, G.; Leus, K.; Couck, S.; Tack, P.; Depauw, H.; Liu, Y.Y.; Vincze, L.; Denayer, J.F.M.; Van Der Voort, P. Enhanced gas sorption and breathing properties of the new sulfone functionalized COMOC-2 metal organic framework. Dalt. Trans. 2016, 45, 9485–9491. [Google Scholar] [CrossRef]
- Wieme, J.; Vanduyfhuys, L.; Rogge, S.M.J.; Waroquier, M.; Van Speybroeck, V. Exploring the flexibility of MIL-47(V)-type materials using force field molecular dynamics simulations. J. Phys. Chem. C 2016, 120, 14934–14947. [Google Scholar] [CrossRef]
- Wang, B.; Xie, L.-H.; Wang, X.; Liu, X.-M.; Li, J.; Li, J.-R. Applications of metal-organic frameworks for green energy and environment: new advances in adsorptive gas separation, storage and removal. Green Energy Environ. 2018, 3, 191–228. [Google Scholar] [CrossRef]
- Lescouet, T.; Kockrick, E.; Pera-titus, M.; Aguado, S.; Farrusseng, D. Homogeneity of flexible metal—Organic frameworks containing mixed linkers. J. Mater. Chem. 2012, 10287–10293. [Google Scholar] [CrossRef]
- Dietzel, P.D.C.; Blom, R.; Fjellvåg, H. Base-induced formation of two magnesium metal-organic framework compounds with a bifunctional tetratopic ligand. Eur. J. Inorg. Chem. 2008, 3624–3632. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Parise, J.B. Recent advances in s-block metal carboxylate networks. Cryst. Growth Des. 2011, 11, 4704–4720. [Google Scholar] [CrossRef]
- Plonka, A.M.; Banerjee, D.; Parise, J.B. Effect of ligand structural isomerism in formation of calcium coordination networks. Cryst. Growth Des. 2012, 12, 2460–2467. [Google Scholar] [CrossRef]
- Ahmad, M.; Sharma, M.K.; Das, R.; Poddar, P.; Bharadwaj, P.K. Syntheses, crystal structures, and magnetic properties of metal-organic hybrid materials of Co(II) using flexible and rigid nitrogen-based ditopic ligands as spacers. Cryst. Growth Des. 2012, 12, 1571–1578. [Google Scholar] [CrossRef]
- Yaghi, O.M.; Li, H.; Davis, C.; Richardson, D.; Groy, T.L. Synthetic strategies, structure patterns, and emerging properties in the chemistry of modular porous solids. Acc. Chem. Res. 1998, 31, 474–484. [Google Scholar] [CrossRef]
- Férey, G.; Serre, C. Large breathing effects in three-dimensional porous hybrid matter: Facts, analyses, rules and consequences. Chem. Soc. Rev. 2009, 38, 1380–1399. [Google Scholar] [CrossRef]
- Seo, J.; Matsuda, R.; Sakamoto, H.; Bonneau, C.; Kitagawa, S. A pillared-layer coordination polymer with a rotatable pillar acting as a molecular gate for guest molecules. J. Am. Chem. Soc. 2009, 131, 12792–12800. [Google Scholar] [CrossRef]
- Henke, S.; Schneemann, A.; Wu, A.; Fischer, R.A. Directing the breathing behavior of pillared-layered metal−organic frameworks via a systematic library of functionalized linkers bearing flexible substituents. J. Am. Chem. Soc. 2012, 134, 9464–9474. [Google Scholar] [CrossRef]
- Biswas, S.; Ahnfeldt, T.; Stock, N. New functionalized flexible Al-MIL-53-X (X= -Cl, -Br, -CH3,-NO2,-(OH)2) solids: syntheses, characterization, sorption, and breathing behavior. Inorg. Chem. 2011, 50, 9518–9526. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Wisser, F.; Bon, V.V.; Grünker, R.; Senkovska, I.; Kaskel, S. Post-synthetic paddle-wheel crosslinking and functionalization of 1,3-phenylenebis(azanetriyl)tetrabenzoate based MOFs. Chem. Mater. 2015, 27, 2460–2467. [Google Scholar] [CrossRef]
- Pan, L.; Frydel, T.; Sander, M.B.; Huang, X.; Li, J. The effect of pH on the dimensionality of coordination polymers. Inorg. Chem. 2001, 40, 1271–1283. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Mellot-Draznieks, C.; Surblé, S.; Audebrand, N.; Filinchuk, Y.; Férey, G. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks. Science 2007, 315, 1828–1831. [Google Scholar] [CrossRef] [PubMed]
- Horcajada, P.; Salles, F.; Wuttke, S.; Devic, T.; Heurtaux, D.; Maurin, G.; Vimont, A.; Daturi, M.; David, O.; Magnier, E.; et al. How linker’s modification controls swelling properties of highly flexible iron(III) dicarboxylates MIL-88. J. Am. Chem. Soc. 2011, 133, 17839–17847. [Google Scholar] [CrossRef] [PubMed]
- Ahnfeldt, T.; Gunzelmann, D.; Loiseau, T.; Hirsemann, D.; Senker, J.; Férey, G.; Stock, N. Synthesis and modification of a functionalized 3D open-framework structure with MIL-53 topology. Inorg. Chem. 2009, 48, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple functional groups of varying ratios in metal-organic frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Neogi, S.; Aijaz, A.; Xu, Q.; Bharadwaj, P.K. Structural variation in Zn(ii) coordination polymers built with a semi-rigid tetracarboxylate and different pyridine linkers: Synthesis and selective CO2 adsorption studies. Dalt. Trans. 2014, 43, 6100–6107. [Google Scholar] [CrossRef]
- Xu, C.; Li, L.; Wang, Y.; Guo, Q.; Wang, X.; Hou, H.; Fan, Y. Three-dimensional Cd(II) coordination polymers based on semirigid bis(methylbenzimidazole) and aromatic polycarboxylates: Syntheses, topological structures and photoluminescent properties. Cryst. Growth Des. 2011, 11, 4667–4675. [Google Scholar] [CrossRef]
- Gupta, A.K.; Tomar, K.; Bharadwaj, P.K. Structural diversity of Zn(II) based coordination polymers constructed from a flexible carboxylate linker and pyridyl co-linkers: Fluorescence sensing of nitroaromatics. New J. Chem. 2017, 41, 14505–14515. [Google Scholar] [CrossRef]
- Hua, X.N.; Qin, L.; Yan, X.Z.; Yu, L.; Xie, Y.X.; Han, L. Conformational diversity of flexible ligand in metal-organic frameworks controlled by size-matching mixed ligands. J. Solid State Chem. 2015, 232, 91–95. [Google Scholar] [CrossRef]
- Yuan, S.; Qin, J.S.; Lollar, C.T.; Zhou, H.C. Stable metal-organic frameworks with group 4 metals: Current status and trends. ACS Cent. Sci. 2018, 4, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Ple´vert, J.; Gentz, T.M.; Laine, A.; Li, H.; Young, V.G.; Yaghi, O.M.; O’Keeffe, M. A Flexible germanate structure containing 24-ring channels and with very low framework density. J. Am. Chem. Soc. 2001, 123, 12706–12707. [Google Scholar] [CrossRef]
- Souza, D.C.S.; Pralong, V.; Jacobson, A.J.; Nazar, L.F. A reversible solid-state crystalline transformation in a metal phosphide induced by redox chemistry. Science 2002, 296, 2012–2015. [Google Scholar] [CrossRef] [PubMed]
- Amos, T.G.; Sleight, A.W. Negative thermal expansion in orthorhombic NbOPO4. J. Solid State Chem. 2001, 160, 230–238. [Google Scholar] [CrossRef]
- Takaishi, T.; Tsutsumi, K.; Chubachi, K.; Matsumoto, A. Adsorption induced phase transition of ZSM-5 by p-xylene. J. Chem. Soc. Faraday Trans. 1998, 94, 601–608. [Google Scholar] [CrossRef]
- Chen, Q.; Chang, Z.; Song, W.C.; Song, H.; Song, H.-B.; Hu, T.L.; Bu, X.H. A controllable gate effect in cobalt(II) organic frameworks by reversible structure transformations. Angew. Chem. Int. Ed. 2013, 52, 11550–11553. [Google Scholar] [CrossRef]
- Tian, J.; Saraf, L.V.; Schwenzer, B.; Taylor, S.M.; Brechin, E.K.; Liu, J.; Dalgarno, S.J.; Thallapally, P.K. Selective metal cation capture by soft anionic metal-organic frameworks via drastic single-crystal-to-single-crystal transformations. J. Am. Chem. Soc. 2012, 134, 9581–9584. [Google Scholar] [CrossRef]
- Seo, J.; Bonneau, C.; Matsuda, R.; Takata, M.; Kitagawa, S. Soft secondary building unit: Dynamic bond rearrangement on multinuclear core of porous coordination polymers in gas media. J. Am. Chem. Soc. 2011, 133, 9005–9013. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhou, H.-L.; Zhou, D.-D.; Liao, P.-Q.; Chen, X.-M. Controlling flexibility of metal–organic frameworks. Natl. Sci. Rev. 2017, 5, 907–919. [Google Scholar] [CrossRef]
- Ogborn, J.M.; Collings, I.E.; Moggach, S.A.; Goodwin, A.L.T.A.L. Supramolecular mechanics in a metal–organic framework. Chem. Sci. 2012, 3, 3011–3017. [Google Scholar] [CrossRef]
- Schneemann, A.; Bon, V.; Schwedler, I.; Senkovska, I.; Kaskel, S.; Fischer, R.A. Flexible metal-organic frameworks. Chem. Soc. Rev. 2014, 43, 6062–6096. [Google Scholar] [CrossRef] [PubMed]
- Millange, F.; Serre, C.; Férey, G. Synthesis, structure determination and properties of {MIL}-53as and {MIL}-53ht:the first Criii(OH)·{O2C-C6H4-CO2}·{HO2C-C6H4-CO2H}x. Chem. Commun. 2002, 8, 822–823. [Google Scholar] [CrossRef]
- Mowat, J.P.S.; Seymour, V.R.; Griffin, J.M.; Thompson, S.P.; Slawin, A.M.Z.; Fairen-Jimenez, D.; Düren, T.; Ashbrook, S.E.; Wright, P.A. A novel structural form of MIL-53 observed for the scandium analogue and its response to temperature variation and CO2 adsorption. Dalt. Trans. 2012, 41, 3937–3941. [Google Scholar] [CrossRef] [PubMed]
- Anokhina, E.V.; Vougo-Zanda, M.; Wang, X.; Jacobson, A.J. In(OH)BDC·0.75BDCH2 (BDC = benzenedicarboxylate), a hybrid inorganic-organic vernier structure. J. Am. Chem. Soc. 2005, 127, 15000–15001. [Google Scholar] [CrossRef]
- Boutin, A.; Springuel-Huet, M.A.; Nossov, A.; Gédéon, A.; Loiseau, T.; Volkringer, C.; Férey, G.; Coudert, F.X.; Fuchs, A.H. Breathing transitions in MIL-53(A1) metal-organic framework upon Xenon adsorption. Angew. Chem. Int. Ed. 2009, 48, 8314–8317. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Yadian, B.; Wu, R.; Long, Y.; Zhou, K.; Zhu, B.; Huang, Y. Structure stability of metal-organic framework MIL-53 (Al) in aqueous solutions. Int. J. Hydrogen Energy 2013, 38, 16710–16715. [Google Scholar] [CrossRef]
- Millange, F.; Guillou, N.; Walton, R.I.; Grenèche, J.M.; Margiolaki, I.; Férey, G. Effect of the nature of the metal on the breathing steps in MOFs with dynamic frameworks. Chem. Commun. 2008, 39, 4732–4734. [Google Scholar] [CrossRef]
- Beurroies, I.; Boulhout, M.; Llewellyn, P.L.; Kuchta, B.; Férey, G.; Serre, C.; Denoyel, R. Using pressure to provoke the structural transition of metal-organic frameworks. Angew. Chem. Int. Ed. 2010, 49, 7526–7529. [Google Scholar] [CrossRef]
- Yot, P.G.; Ma, Q.; Haines, J.; Yang, Q.; Ghoufi, A.; Devic, T.; Serre, C.; Dmitriev, V.; Férey, G.; Zhong, C.; et al. Large breathing of the MOF MIL-47(VIV) under mechanical pressure: A joint experimental-modelling exploration. Chem. Sci. 2012, 3, 1100–1104. [Google Scholar] [CrossRef]
- Clearfield, A. Flexible MOFs under stress: Pressure and temperature. Dalt. Trans. 2016, 45, 4100–4112. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.A.; Thallapally, P.K.; McGrail, B.P. Insights into the temperature-dependent “breathing” of a flexible fluorinated metal-organic framework. ChemPhysChem 2012, 13, 3275–3281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.P.; Chen, X.M. Optimized acetylene/carbon dioxide sorption in a dynamic porous crystal. J. Am. Chem. Soc. 2009, 131, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.C.; Kiran, M.S.R.N.; Li, W.; Ramamurty, U.; Ross, N.L.; Cheetham, A.K. Pressure-induced bond rearrangement and reversible phase transformation in a metal-organic framework. Angew. Chem. Int. Ed. 2014, 53, 5583–5586. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.J.; Beavers, C.M.; Clearfield, A. MOFs under pressure: The reversible compression of a single crystal. J. Am. Chem. Soc. 2013, 135, 1252–1255. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional hybrid porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Medishetty, R.; Fischer, R.A. Guest molecules as a design element for metal-organic frameworks. MRS Bull. 2016, 41, 865–869. [Google Scholar] [CrossRef]
- Yanai, N.; Uemura, T.; Inoue, M.; Matsuda, R.; Fukushima, T.; Tsujimoto, M.; Isoda, S.; Kitagawa, S. Guest-to-host transmission of structural changes for stimuli-responsive adsorption property. J. Am. Chem. Soc. 2012, 134, 4501–4504. [Google Scholar] [CrossRef]
- Luo, F.; Fan, C.-B.; Luo, M.B.; Wu, X.L.; Zhu, Y.; Pu, S.Z.; Xu, W.Y.; Guo, G.C. Photoswitching CO2 capture and release in a photochromic diarylethene metal-organic framework. Angew. Chem. Int. Ed. 2014, 53, 9298–9301. [Google Scholar] [CrossRef]
- Park, J.; Yuan, D.; Pham, K.T.; Li, J.-R.; Yakovenko, A.; Zhou, H.-C. Reversible alteration of CO2 adsorption upon photochemical or thermal treatment in a metal−organic framework. J. Am. Chem.Soc. 2012, 134, 99–10212. [Google Scholar] [CrossRef]
- Kitagawa, S.; Uemura, K. Dynamic porous properties of coordination polymers inspired by hydrogen bonds. Chem. Soc. Rev. 2005, 34, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Kaskel, S. The Chemistry of Metal–Organic Frameworks; Kaskel, S., Ed.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007; Volume 1, ISBN 9781118379028. [Google Scholar]
- Coudert, F.X.; Boutin, A.; Jeffroy, M.; Mellot-Draznieks, C.; Fuchs, A.H. Thermodynamic methods and models to study flexible metal-organic frameworks. ChemPhysChem 2011, 12, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Murdock, C.R.; Hughes, B.C.; Lu, Z.; Jenkins, D.M. Approaches for synthesizing breathing MOFs by exploiting dimensional rigidity. Coord. Chem. Rev. 2014, 258–259, 119–136. [Google Scholar] [CrossRef]
- Song, H.; Jing, C.; Ma, W.; Xie, T.; Long, Y.-T. Reversible photoisomerization of azobenzene molecules on single gold nanoparticle surface. Chem. Commun. 2016, 52, 2984–2987. [Google Scholar] [CrossRef] [PubMed]
- Kanoo, P.; Matsuda, R.; Higuchi, M.; Kitagawa, S.; Maji, T.K. New interpenetrated copper coordination polymer frameworks having porous properties. Chem. Mater. 2009, 21, 5860–5866. [Google Scholar] [CrossRef]
- Maji, T.K.; Matsuda, R.; Kitagawa, S. A flexible interpenetrating coordination framework with a bimodal porous functionality. Nat. Mater. 2007, 6, 142–148. [Google Scholar] [CrossRef]
- Kitaura, R.; Kitagawa, S.; Kubota, Y.; Kobayashi, T.C.; Kindo, K.; Mita, Y.; Matsuo, A.; Kobayashi, M.; Chang, H.C.; Ozawa, T.C.; et al. Formation of a one-dimensional array of oxygen in a microporous metal-organic solid. Science 2002, 298, 2358–2361. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E.J.; McAnally, C.A.; Fletcher, A.J.; Thompson, S.P.; Warren, M.; Brammer, L. Solvent-switchable continuous-breathing behaviour in a diamondoid metal-organic framework and its influence on CO2 versus CH4 selectivity. Nat. Chem. 2017, 9, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Maspoch, D.; Ruiz-Molina, D.; Wurst, K.; Domingo, N.; Cavallini, M.; Biscarini, F.; Tejada, J.; Rovira, C.; Veciana, J. A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties. Nat. Mater. 2003, 2, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Serre, C.; Millange, F.; Thouvenot, C.; Noguès, M.; Marsolier, G.; Loue¨r, D.; Ferey, G. Very large breathing effect in the first nanoporous chromium(III)-based solids: MIL-53 or CrIII (OH)‚{O2C-C6H4 -CO2 }‚{HO2C-C6H2 -CO4H}x‚H2O. J. Am. Chem. Soc. 2002, 124, 13519–13526. [Google Scholar] [CrossRef] [PubMed]
- Suh, M.P. Metal-Organic frameworks and porous coordination polymers: properties and applications. Bull. Jpn. Soc. Coord. Chem. 2015, 65, 9–22. [Google Scholar] [CrossRef]
- Carlucci, L.; Ciani, G.; Moret, M.; Proserpio, D.M.; Rizzato, S. Polymeric layers catenated by ribbons of rings in a three-dimensional self-assembled architecture: A nanoporous network with spongelike behavior. Angew. Chem. Int. Ed. 2000, 39, 1506–1510. [Google Scholar] [CrossRef]
- Llewellyn, P.L.; Bourrelly, S.; Serre, C.; Filinchuk, Y.; Férey, G. How hydration drastically improves adsorption selectivity for CO2 over CH4 in the flexible chromium terephthalate MIL-53. Angew. Chem. Int. Ed. 2006, 45, 7751–7754. [Google Scholar] [CrossRef] [PubMed]
- Mellot-Draznieks, C.; Serre, C.; Surblé, S.; Audebrand, N.; Férey, G. Very large swelling in hybrid frameworks: A combined computational and powder diffraction study. J. Am. Chem. Soc. 2005, 127, 16273–16278. [Google Scholar] [CrossRef]
- Ramsahye, N.A.; Trung, T.K.; Scott, L.; Nouar, F.; Devic, T.; Horcajada, P.; Magnier, E.; David, O.; Serre, C.; Trens, P. Impact of the flexible character of MIL-88 iron(III) dicarboxylates on the adsorption of n-alkanes. Chem. Mater. 2013, 25, 479–488. [Google Scholar] [CrossRef]
- An, T.; Wang, Y.; Tang, J.; Wang, Y.; Zhang, L.; Zheng, G. A flexible ligand-based wavy layered metal-organic framework for lithium-ion storage. J. Colloid Interface Sci. 2015, 445, 320–325. [Google Scholar] [CrossRef]
- Yan, Y.; Kolokolov, D.I.; Da Silva, I.; Stepanov, A.G.; Blake, A.J.; Dailly, A.; Manuel, P.; Tang, C.C.; Yang, S.; Schröder, M. Porous metal-organic polyhedral frameworks with optimal molecular dynamics and pore geometry for methane storage. J. Am. Chem. Soc. 2017, 139, 13349–13360. [Google Scholar] [CrossRef]
- Fairen-Jimenez, D.; Moggach, S.A.; Wharmby, M.T.; Wright, P.A.; Parsons, S.; Düren, T. Opening the gate: Framework flexibility in ZIF-8 explored by experiments and simulations. J. Am. Chem. Soc. 2011, 133, 8900–8902. [Google Scholar] [CrossRef]
- Casco, M.E.; Cheng, Y.Q.; Daemen, L.L.; Fairen-Jimenez, D.; Ramos-Fernández, E.V.; Ramirez-Cuesta, A.J.; Silvestre-Albero, J. Gate-opening effect in ZIF-8: The first experimental proof using inelastic neutron scattering. Chem. Commun. 2016, 52, 3639–3642. [Google Scholar] [CrossRef]
- Yang, C.; Wang, X.; Omary, M.A. Crystallographic observation of dynamic gas adsorption sites and thermal expansion in a breathable fluorous metal-organic framework. Angew. Chem. Int. Ed. 2009, 48, 2500–2505. [Google Scholar] [CrossRef]
- Alaghemandi, M.; Schmid, R. Model study of thermoresponsive behavior of metal-organic frameworks modulated by linker functionalization. J. Phys. Chem. C 2016, 120, 6835–6841. [Google Scholar] [CrossRef]
- Henke, S.; Schneemann, A.; Fischer, R.A. Massive anisotropic thermal expansion and thermo-responsive breathing in metal-organic frameworks modulated by linker functionalization. Adv. Funct. Mater. 2013, 23, 5990–5996. [Google Scholar] [CrossRef]
- McKellar, S.C.; Moggach, S.A. Structural studies of metal-organic frameworks under high pressure. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Graham, A.J.; Banu, A.M.; Düren, T.; Greenaway, A.; McKellar, S.C.; Mowat, J.P.S.; Ward, K.; Wright, P.A.; Moggach, S.A. Stabilization of scandium terephthalate MOFs against reversible amorphization and structural phase transition by guest uptake at extreme pressure. J. Am. Chem. Soc. 2014, 136, 8606–8613. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Bennett, T.D.; Casati, N.P.M.; Lampronti, G.I.; Moggach, S.A.; Redfern, S.A.T. Pressure-induced oversaturation and phase transition in zeolitic imidazolate frameworks with remarkable mechanical stability. Dalt. Trans. 2015, 44, 4498–4503. [Google Scholar] [CrossRef] [Green Version]
- McKellar, S.C.; Sotelo, J.; Greenaway, A.; Mowat, J.P.S.; Kvam, O.; Morrison, C.A.; Wright, P.A.; Moggach, S.A. Pore shape modification of a microporous metal-organic framework using high pressure: Accessing a new phase with oversized guest molecules. Chem. Mater. 2016, 28, 466–473. [Google Scholar] [CrossRef]
- Chapman, K.W.; Halder, G.J.; Halder, G.J.; Chupas, P.J. Pressure-induced amorphization and porosity modification in a metal-organic framework. J. Am. Chem. Soc. 2009, 131, 17546–17547. [Google Scholar] [CrossRef]
- Chapman, K.W.; Halder, G.J.; Chupas, P.J. Guest-dependent high pressure phenomena in a nanoporous metal-organic framework material. J. Am. Chem. Soc. 2008, 130, 10524–10526. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Banerjee, D.; Thallapally, P.K. Flexibility in metal – organic frameworks: A fundamental understanding. Coord. Chem. Rev. 2018, 358, 125–152. [Google Scholar] [CrossRef]
- Yuan, S.; Sun, X.; Pang, J.; Lollar, C.; Qin, J.S.; Perry, Z.; Joseph, E.; Wang, X.; Fang, Y.; Bosch, M.; et al. PCN-250 under pressure: Sequential phase transformation and the implications for MOF densification. Joule 2017, 1, 806–815. [Google Scholar] [CrossRef]
- Serra-Crespo, P.; Dikhtiarenko, A.; Stavitski, E.; Juan-Alcañiz, J.; Kapteijn, F.; Coudert, F.X.; Gascon, J. Experimental evidence of negative linear compressibility in the MIL-53 metal-organic framework family. CrystEngComm 2015, 17, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Probert, M.R.; Kosa, M.; Bennett, T.D.; Thirumurugan, A.; Burwood, R.P.; Parinello, M.; Howard, J.A.K.; Cheetham, A.K. Negative linear compressibility of a metal-organic framework. J. Am. Chem. Soc. 2012, 134, 11940–11943. [Google Scholar] [CrossRef] [PubMed]
- Coudert, F.X. Responsive metal-organic frameworks and framework materials: Under pressure, taking the heat, in the spotlight, with friends. Chem. Mater. 2015, 27, 1905–1916. [Google Scholar] [CrossRef]
- Lyndon, R.; Konstas, K.; Ladewig, B.P.; Southon, P.D.; Kepert, P.C.J.; Hill, M.R. Dynamic photo-switching in metal-organic frameworks as a route to low-energy carbon dioxide capture and release. Angew. Chem. Int. Ed. 2013, 52, 3695–3698. [Google Scholar] [CrossRef] [PubMed]
- Modrow, A.; Zargarani, D.; Herges, R.; Stock, N. The first porous MOF with photoswitchable linker molecules. Dalt. Trans. 2011, 40, 4217–4222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermann, D.; Emerich, H.; Lepski, R.; Schaniel, D.; Ruschewitz, U. Metal-organic frameworks as hosts for photochromic guest molecules. Inorg. Chem. 2013, 52, 2744–2749. [Google Scholar] [CrossRef]
- Modrow, A.; Zargarani, D.; Herges, R.; Stock, N. Introducing a photo-switchable azo-functionality inside Cr-MIL-101-NH2 by covalent post-synthetic modification. Dalt. Trans. 2012, 41, 8690–8696. [Google Scholar] [CrossRef]
- Bernt, S.; Feyand, M.; Modrow, A.; Wack, J.; Senker, J.; Stock, N. A mixed-linker ZIF containing a photoswitchable phenylazo group. Eur. J. Inorg. Chem. 2011, 5378–5383. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.D.; Praveen, V.K.; Ajayaghosh, A. Photoresponsive metal-organic materials: Exploiting the azobenzene switch. Mater. Horizons 2014, 1, 572–576. [Google Scholar] [CrossRef]
- Lu, Y.M.; Lan, Y.Q.; Xu, Y.H.; Su, Z.M.; Li, S.L.; Zang, H.Y.; Xu, G.J. Interpenetrating metal-organic frameworks formed by self-assembly of tetrahedral and octahedral building blocks. J. Solid State Chem. 2009, 182, 3105–3112. [Google Scholar] [CrossRef]
- Zou, Y.; Park, M.; Hong, S.; Lah, M.S. A designed metal-organic framework based on a metal-organic polyhedron. Chem. Commun. 2008, 20, 2340–2342. [Google Scholar] [CrossRef] [PubMed]
- Deria, P.; Bury, W.; Hupp, J.T.; Farha, O.K. Versatile functionalization of the NU-1000 platform by solvent-assisted ligand incorporation. Chem. Commun. 2014, 50, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, M.; Howarth, A.J.; Destefano, M.R.; Lin, L.; Goswami, S.; Li, P.; Hupp, J.T.; Farha, O.K. Catalytic zirconium/hafnium-based metal-organic frameworks. ACS Catal. 2017, 7, 997–1014. [Google Scholar] [CrossRef]
- Deria, P.; Chung, Y.G.; Snurr, R.Q.; Hupp, J.T.; Farha, O.K. Water stabilization of Zr6-based metal–organic frameworks via solvent-assisted ligand incorporation. Chem. Sci. 2015, 6, 5172–5176. [Google Scholar] [CrossRef] [PubMed]
- Deria, P.; Bury, W.; Hod, I.; Kung, C.; Karagiaridi, O.; Hupp, J.T.; Farha, O.K. MOF functionalization via solvent-assisted ligand incorporation: Phosphonates vs carboxylates. Inorg. Chem. 2015, 54, 2185–2198. [Google Scholar] [CrossRef]
- Hod, I.; Bury, W.; Gardner, D.M.; Deria, P.; Roznyatovskiy, V.; Wasielewski, M.R.; Farha, O.K.; Hupp, J.T. Bias-switchable permselectivity and redox catalytic activity of a ferrocene-functionalized, thin-film metal−organic framework compound. J. Phys. Chem. Lett. 2015, 6, 586–591. [Google Scholar] [CrossRef]
- Taddei, M.; Costantino, F.; Ienco, A.; Comotti, A.; Dau, P.V.; Cohen, S.M. Synthesis, breathing, and gas sorption study of the first isoreticular mixed-linker phosphonate based metal–organic frameworks. Chem. Commun 2013, 49, 1315–1317. [Google Scholar] [CrossRef]
- Vermoortele, F.; Vandichel, M.; Van De Voorde, B.; Ameloot, R.; Waroquier, M.; Van Speybroeck, V.; De Vos, D.E. Electronic effects of linker substitution on Lewis acid catalysis with metal-organic frameworks. Angew. Chem. Int. Ed. 2012, 51, 4887–4890. [Google Scholar] [CrossRef]
- Horike, S.; Shimomura, S.; Kitagawa, S. Soft porous crystals. Nat. Chem. 2009, 1, 695–704. [Google Scholar] [CrossRef]
- Devic, T.; Horcajada, P.; Serre, C.; Salles, F.; Maurin, G.; Heurtaux, D.; Clet, G.; Vimont, A.; Grene, J.; Le Ouay, B.; et al. Functionalization in flexible porous Solids: Effects on the Pore Opening and the Host - Guest Interactions. J. Am. Chem. Soc. 2010, 1127–1136. [Google Scholar] [CrossRef]
- Serre, C.; Millange, F.; Devic, T.; Audebrand, N.; Beek, W. Van Synthesis and structure determination of new open-framework chromium carboxylate MIL-105 or Cr (OH).{O2C–C6( CH3)4 – CO2}.nH2O. Mater. Res. Bull. 2006, 41, 1550–1557. [Google Scholar] [CrossRef]
- Serra-Crespo, P.; Van Der Veen, M.A.; Gobechiya, E.; Houthoofd, K.; Filinchuk, Y.; Kirschhock, C.E.A.; Martens, J.A.; Sels, B.F.; De Vos, D.E.; Kapteijn, F.; et al. NH2-MIL-53(Al): A high-contrast reversible solid-state nonlinear optical switch. J. Am. Chem. Soc. 2012, 134, 8314–8317. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Surble, S.; Serre, C.; Mellot-draznieks, C. A new isoreticular class of metal-organic-frameworks with the MIL-88 topology. Chem. Commun. 2006, 3, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Bon, V.; Kavoosi, N.; Senkovska, I.; Müller, P.; Schaber, J.; Wallacher, D.; Többens, D.M.; Mueller, U.; Kaskel, S. Tuning the flexibility in MOFs by SBU. Dalt. Trans. 2016, 45, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- Salles, F.; Maurin, G.; Serre, C.; Llewellyn, P.L.; Knöfel, C.; Choi, H.J.; Filinchuk, Y.; Oliviero, L.; Vimont, A.; Long, J.R.; et al. Multistep N2 breathing in the metal-organic framework Co(1,4-benzenedipyrazolate). J. Am. Chem. Soc. 2010, 132, 13782–13788. [Google Scholar] [CrossRef] [PubMed]
- Thallapally, P.K.; Tian, J.; Kishan, M.R.; Fernandez, C.A.; Dalgarno, S.J.; McGrail, P.B.; Warren, J.E.; Atwood, J.L. Flexible (breathing) interpenetrated metal-organic frameworks for CO2 separation applications. J. Am. Chem. Soc. 2008, 130, 16842–16843. [Google Scholar] [CrossRef]
- Greathouse, J.A.; Allendorf, M.D. The interaction of water with MOF-5 simulated by molecular dynamics. J. Am. Chem. Soc. 2006, 128, 10678–10679. [Google Scholar] [CrossRef]
- Springuel-Huet, M.-A.; Nossov, A.; Adem, Z.; Guenneau, F.; Volkringer, C.; Loiseau, T.; Ge, A.; Marie, P. Xe NMR study of the framework flexibility of the porous hybrid MIL-53(Al). JACS 2010, 53, 11599–11607. [Google Scholar] [CrossRef]
- Gould, S.L.; Tranchemontagne, D.; Yaghi, O.M.; Garcia-Garibay, M.A. Amphidynamic character of crystalline MOF-5: Rotational dynamics of terephthalate phenylenes in a free-volume, sterically unhindered environment. J. Am. Chem. Soc. 2008, 130, 3246–3247. [Google Scholar] [CrossRef]
- Horike, S.; Matsuda, R.; Tanaka, D.; Matsubara, S.; Mizuno, M.; Endo, K.; Kitagawa, S. Dynamic motion of building blocks in porous coordination polymers. Angew. Chem. Int. Ed. 2006, 45, 7226–7230. [Google Scholar] [CrossRef] [PubMed]
- Winston, E.B.; Lowell, P.J.; Vacek, J.; Chocholoušová, J.; Michl, J.; Price, J.C. Dipolar molecular rotors in the metal-organic framework crystal IRMOF-2. Phys. Chem. Chem. Phys. 2008, 10, 5188–5191. [Google Scholar] [CrossRef] [PubMed]
- Devic, T.; Salles, F.; Bourrelly, S.; Moulin, B.; Maurin, G.; Horcajada, P.; Serre, C.; Vimont, A.; Lavalley, J.-C.; Leclerc, H.; et al. Effect of the organic functionalization of flexible MOFs on the adsorption of CO2. J. Mater. Chem. 2012, 22, 10266–10273. [Google Scholar] [CrossRef]
- Hamon, L.; Llewellyn, P.L.; Devic, T.; Ghoufi, A.; Clet, G.; Guillerm, V.; Pirngruber, G.D.; Maurin, G.; Serre, C.; Driver, G.; et al. Co-adsorption and separation of CO2 - CH4 mixtures in the highly flexible MIL-53(Cr) MOF. JACS 2009, 53, 17490–17499. [Google Scholar] [CrossRef] [PubMed]
- Quartapelle Procopio, E.; Fukushima, T.; Barea, E.; Navarro, J.A.R.; Horike, S.; Kitagawa, S. A soft copper(II) porous coordination polymer with unprecedented aqua bridge and selective adsorption properties. Chem. A Eur. J. 2012, 18, 13117–13125. [Google Scholar] [CrossRef] [PubMed]
- Horike, S.; Inubushi, Y.; Hori, T.; Fukushima, T.; Kitagawa, S. A solid solution approach to 2D coordination polymers for CH4/CO2 and CH4/C2H6 gas separation: Equilibrium and kinetic studies. Chem. Sci. 2012, 3, 116–120. [Google Scholar] [CrossRef]
- Kim, J.; Kim, W.Y.; Ahn, W.S. Amine-functionalized MIL-53(Al) for CO2/N2 separation: Effect of textural properties. Fuel 2012, 102, 574–579. [Google Scholar] [CrossRef]
- Chen, B.; Xiang, S.; Qian, G. Metal-organic frameworks with functional pores for recognition of small molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef]
- Das, R.K.; Aijaz, A.; Sharma, M.K.; Lama, P.; Bharadwaj, P.K. Direct crystallographic observation of catalytic reactions inside the pores of a flexible coordination polymer. Chem. A Eur. J. 2012, 18, 6866–6872. [Google Scholar] [CrossRef]
- Klein, N.; Herzog, C.; Sabo, M.; Senkovska, I.; Getzschmann, J.; Paasch, S.; Lohe, M.R.; Brunner, E.; Kaskel, S. Monitoring adsorption-induced switching by129Xe NMR spectroscopy in a new metal-organic framework Ni2 (2,6-ndc)2 (dabco). Phys. Chem. Chem. Phys. 2010, 12, 11778–11784. [Google Scholar] [CrossRef]
- Horcajada, P.; Serre, C.; Maurin, G.; Ramsahye, N.A.; Balas, F.; Vallet-Regí, M.; Sebban, M.; Taulelle, F.; Férey, G. Flexible porous metal-organic frameworks for a controlled drug delivery. J. Am. Chem. Soc. 2008, 130, 6774–6780. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Masel, R.I. Water repellent metal-organic frameworks, process for making and uses regarding same. U.S. Patent 8,269,029, 18 September 2012. [Google Scholar]
- Leung, E.; Müller, U.; Trukhan, N.; Cox, G.; Mattenheimer, H.; Blei, S. Process for preparing porous metal-organic frameworks based on aluminum fumarate. U.S. Patent 8,524,932, 3 September 2013. [Google Scholar]
- Stavitski, E.; Pidko, E.A.; Couck, S.; Remy, T.; Hensen, E.J.M.; Weckhuysen, B.M.; Denayer, J.; Gascon, J.; Kapteijn, F. Complexity behind CO2 capture on NH2-MIL-53(Al). Langmuir 2011, 27, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Gehre, M.; Guo, Z.; Rothenberg, G.; Tanase, S. Sustainable separations of C4-hydrocarbons by using microporous materials. ChemSusChem 2017, 10, 3947–3963. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lin, R.B.; Krishna, R.; Wang, X.; Li, B.; Wu, H.; Li, J.; Zhou, W.; Chen, B. Flexible-robust metal-organic framework for efficient removal of propyne from propylene. J. Am. Chem. Soc. 2017, 139, 7733–7736. [Google Scholar] [CrossRef] [PubMed]
- El Osta, R.; Carlin-Sinclair, A.; Guillou, N.; Walton, R.I.; Vermoortele, F.; Maes, M.; De Vos, D.; Millange, F. Liquid-phase adsorption and separation of xylene isomers by the flexible porous metal-organic framework MIL-53(Fe). Chem. Mater. 2012, 24, 2781–2791. [Google Scholar] [CrossRef]
- Allen, A.J.; Espinal, L.; Wong-Ng, W.; Queen, W.L.; Brown, C.M.; Kline, S.R.; Kauffman, K.L.; Culp, J.T.; Matranga, C. Flexible metal-organic framework compounds: In situ studies for selective CO2 capture. J. Alloys Compd. 2015, 647, 24–34. [Google Scholar] [CrossRef]
- Yanai, N.; Kitayama, K.; Hijikata, Y.; Sato, H.; Matsuda, R.; Kubota, Y.; Takata, M.; Mizuno, M.; Uemura, T.; Kitagawa, S. Gas detection by structural variations of fluorescent guest molecules in a flexible porous coordination polymer. Nat. Mater. 2011, 10, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.Y.; Huang, H.L.; Wang, J.Y.; Li, H.Y.; Zang, S.Q. A Flexible fluorescent SCC-MOF for switchable molecule identification and temperature display. Chem. Mater. 2018, 30, 2160–2167. [Google Scholar] [CrossRef]





















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljammal, N.; Jabbour, C.; Chaemchuen, S.; Juzsakova, T.; Verpoort, F. Flexibility in Metal–Organic Frameworks: A Basic Understanding. Catalysts 2019, 9, 512. https://doi.org/10.3390/catal9060512
Aljammal N, Jabbour C, Chaemchuen S, Juzsakova T, Verpoort F. Flexibility in Metal–Organic Frameworks: A Basic Understanding. Catalysts. 2019; 9(6):512. https://doi.org/10.3390/catal9060512
Chicago/Turabian StyleAljammal, Noor, Christia Jabbour, Somboon Chaemchuen, Tatjána Juzsakova, and Francis Verpoort. 2019. "Flexibility in Metal–Organic Frameworks: A Basic Understanding" Catalysts 9, no. 6: 512. https://doi.org/10.3390/catal9060512
APA StyleAljammal, N., Jabbour, C., Chaemchuen, S., Juzsakova, T., & Verpoort, F. (2019). Flexibility in Metal–Organic Frameworks: A Basic Understanding. Catalysts, 9(6), 512. https://doi.org/10.3390/catal9060512