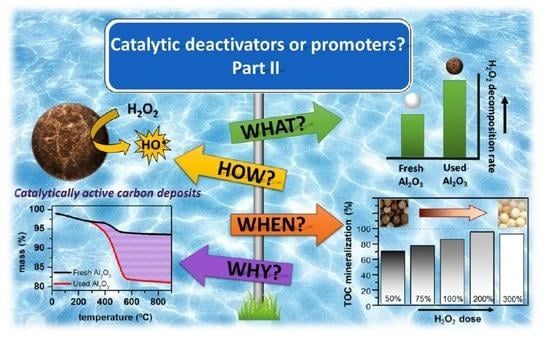
Condensation By-Products in Wet Peroxide Oxidation: Fouling or Catalytic Promotion? Part II: Activity, Nature and Stability
Abstract
:1. Introduction
2. Results and Discussion
2.1. Catalytic Activity Promoted by Condensation By-Products Deposits
2.2. Nature of the Condensation By-Product Deposits
2.3. Kinetic Modeling
2.4. Stability of the Condensation By-Product Deposits
3. Experimental Methods
3.1. Al2O3 Spheres and Characterization
3.2. Oxidation Experiments
3.3. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Delgado, J.J.; Chen, X.; Pérez-Omil, J.A.; Rodríguez-Izquierdo, J.M.; Cauqui, M.A. The effect of reaction conditions on the apparent deactivation of Ce–Zr mixed oxides for the catalytic wet oxidation of phenol. Catal. Today 2012, 180, 25–33. [Google Scholar] [CrossRef]
- Vallejo, M.; Fernández-Castro, P.; San Román, M.F.; Ortiz, I. Assessment of PCDD/Fs formation in the Fenton oxidation of 2-chlorophenol: Influence of the iron dose applied. Chemosphere 2015, 137, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, C.M.; Ocón, P.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Highly efficient application of activated carbon as catalyst for wet peroxide oxidation. Appl. Catal. B 2013, 140–141, 663–670. [Google Scholar] [CrossRef]
- Poerschmann, J.; Trommler, U.; Górecki, T.; Kopinke, F.D. Formation of chlorinated biphenyls, diphenyl ethers and benzofurans as a result of Fenton-driven oxidation of 2-chlorophenol. Chemosphere 2009, 75, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Assessment of the generation of chlorinated byproducts upon Fenton-like oxidation of chlorophenols at different conditions. J. Hazard. Mater. 2011, 190, 993–1000. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Tuesta, J.L.; Quintanilla, A.; Casas, J.A.; Rodriguez, J.J. Kinetic modeling of wet peroxide oxidation with a carbon black catalyst. Appl. Catal. B 2017, 209, 701–710. [Google Scholar] [CrossRef]
- Munoz, M.; de Pedro, Z.M.; Casas, J.A.; Rodriguez, J.J. Combining efficiently catalytic hydrodechlorination and wet peroxide oxidation (HDC–CWPO) for the abatement of organochlorinated water pollutants. Appl. Catal. B 2014, 150–151, 197–203. [Google Scholar] [CrossRef]
- Hamoudi, S.; Larachi, F.; Adnot, A.; Sayari, A. Characterization of Spent MnO2/CeO2 Wet Oxidation Catalyst by TPO–MS, XPS, and S-SIMS. J. Catal. 1999, 185, 333–344. [Google Scholar] [CrossRef]
- Kim, S.; Ihm, S. Nature of carbonaceous deposits on the alumina supported transition metal oxide catalysts in the wet air oxidation of phenol. Top. Catal. 2005, 33, 171–179. [Google Scholar] [CrossRef]
- Lee, D.K.; Kim, D.S.; Kim, T.H.; Lee, Y.K.; Jeong, S.E.; Le, N.T.; Cho, M.J.; Henam, S.D. Deactivation of Pt catalysts during wet oxidation of phenol. Catal. Today 2010, 154, 244–249. [Google Scholar] [CrossRef]
- Keav, S.; de los Monteros, A.E.; Barbier, J.; Duprez, D. Wet Air Oxidation of phenol over Pt and Ru catalysts supported on cerium-based oxides: Resistance to fouling and kinetic modelling. Appl. Catal. B 2014, 150–151, 402–410. [Google Scholar] [CrossRef]
- Yadav, A.; Verma, N. Carbon bead-supported copper-dispersed carbon nanofibers: An efficient catalyst for wet air oxidation of industrial wastewater in a recycle flow reactor. J. Ind. Eng. Chem. 2018, 67, 448–460. [Google Scholar] [CrossRef]
- Catrinescu, C.; Teodosiu, C.; Macoveanu, M.; Miehe-Brendlé, J.; Le Dred, R. Catalytic wet peroxide oxidation of phenol over Fe-exchanged pillared beidellite. Water Res. 2003, 37, 1154–1160. [Google Scholar] [CrossRef]
- Collett, C.H.; McGregor, J. Things go better with coke: the beneficial role of carbonaceous deposits in heterogeneous catalysis. Catal. Sci. Technol. 2016, 6, 363–378. [Google Scholar] [CrossRef]
- Catrinescu, C.; Arsene, D.; Teodosiu, C. Catalytic wet hydrogen peroxide oxidation of para-chlorophenol over Al/Fe pillared clays (AlFePILCs) prepared from different host clays. Appl. Catal. B 2011, 101, 451–460. [Google Scholar] [CrossRef]
- Quintanilla, A.; Díaz de Tuesta, J.L.; Figueruelo, C.; Munoz, M.; Casas, J.A. Condensation by-products in wet peroxide oxidation: fouling or catalytic promotion? Part I. Evidences of an autocatalytic process. Catalysts 2019, in press. [Google Scholar]
- Becerra, M.E.; Arias, N.P.; Giraldo, O.H.; López-Suárez, F.E.; Illán-Gómez, M.J.; Bueno-López, A. Alumina-supported manganese catalysts for soot combustion prepared by thermal decomposition of KMnO4. Catalysts 2012, 2, 352–357. [Google Scholar] [CrossRef]
- Lefävre, G.; Duc, M.; Lepeut, P.; Caplain, R.; Fadoroff, M. Hydration of Î3-alumina in water and its effects on surface reactivity. Langmuir 2002, 18, 7530–7537. [Google Scholar] [CrossRef]
- El-Naggar, A.Y. Thermal analysis of the modified, coated and bonded alumina surfaces. J. Emer. Trends Eng. Appl. Sci. 2014, 5, 30–34. [Google Scholar]
- Ram, S. Infrared spectral study of molecular vibrations in amorphous, nanocrystalline and AlO(OH)·αH2O bulk crystals. Infrared Phys. Technol. 2001, 42, 547–560. [Google Scholar] [CrossRef]
- Nyquist, R.A.; Lengers, M.A. Handbook of infrared and ramen spectra of inorganic compounds and organic salts. Search PubMed 1997, 4, 72. [Google Scholar]
- Lambert, J.B.; Shurvell, H.F.; Lightner, D.A.; Cooks, R.G. Introduction to Organic Spectroscopy; Macmillan Publishing Company: London, UK, 1987. [Google Scholar]
- Estrade-Szwarckopf, H. XPS photoemission in carbonaceous materials: A “defect” peak beside the graphitic asymmetric peak. Carbon 2004, 42, 1713–1721. [Google Scholar] [CrossRef]
- Ishimaru, K.; Hata, T.; Bronsveld, P.; Meier, D.; Imamura, Y. Spectroscopic analysis of carbonization behavior of wood, cellulose and lignin. J. Mater. Sci. 2007, 42, 122–129. [Google Scholar] [CrossRef]
- Quintanilla, A.; Casas, J.A.; Zazo, J.A.; Mohedano, A.F.; Rodríguez, J.J. Wet air oxidation of phenol at mild conditions with a Fe/activated carbon catalyst. Appl. Catal. B 2006, 62, 115–120. [Google Scholar] [CrossRef]










| Process | Catalyst | Target Pollutant | Operating Conditions | Condensation Products | Reference |
|---|---|---|---|---|---|
| CWAO | MnO2/CeO2 | Phenol | T = 80–130 °C PO2 = 5 bar Ccat = 1–5 g L−1 Ccont = 1–10 g L−1 | Formation: A carbonaceous overlayer was formed upon CWAO. The amount of surface carbon increased with reaction time, whereas its composition was reaction time and temperature dependent. Characterization: TPO-MS, XPS and S-SIMS. Low polycondensation (4 condensed aromatic rings) deposits were formed. The presence of aliphatic species as well as oxygen-containing compounds from alcohol/ether origin was also evidenced. Regeneration: oxidation in air (200–300 °C). | [8] |
| CWAO | Mn/Al2O3 Fe/Al2O3 Co/Al2O3 Ni/Al2O3 Cu/Al2O3 | Phenol | T = 150 °C Pair = 20 bar Ccat = 3 g L−1 Ccont = 1 g L−1 | Formation: Used catalysts showed a darker color due to the formation of carbonaceous deposits. Mn/Al2O3 showed the highest amount of deposits. Characterization: Specific surface area, NMR and FTIR. The carbonaceous deposits showed a microporous structure, increasing the surface area of the catalyst. They were mostly of aromatic nature, containing also oxygen-bearing groups such as carboxylic acids and alcohols. Regeneration: N/A | [9] |
| CWAO | Pt/Al2O3 Pt/CeO2 | Phenol | T = 150 °C PO2 = 14 bar Ccat = 1–3 g L−1 Ccont = 0.5–10 g L−1 | Formation: Along reaction, carbonaceous deposits were formed on the catalyst surface, leading to its deactivation, especially with Pt/Al2O3. Characterization: TEM, EDX, XPS and TPO. The carbonaceous deposits were predominantly formed on the surface of Pt particles. The deposits were burned at 250–700 °C in Pt/Al2O3 and 250–450 °C in Pt/CeO2. Regeneration: N/A | [10] |
| CWAO | Ce–Zr mixed oxides | Phenol | T = 120–160 °C PO2 = 5–20 bar Ccat = 1.9–5.8 g L–1 Ccont = 0.25–3.8 g L–1 | Formation: Phenolic polymers are formed and further degraded along reaction depending on the operating conditions. At 120 °C, their formation is favored, leading to a deactivation of the catalyst. At 160 °C, the deposits are completely oxidized to CO2. Characterization: Quantification by carbon-mass balances, TPO and specific surface area. The carbon deposits amounted from 2.9% to 30% wt. They were burn-off at mild temperatures (<350 °C). The specific surface area was slightly decreased due to carbon deposits coverage (1%–13%). Regeneration: Calcination at 350 °C. | [1] |
| CWAO | Ru/Ce, ZrCe or ZrCePr Pt/Ce, ZrCe or ZrCePr | Phenol | T = 160 °C PO2 = 20 bar Ccat = 4 g L−1 Ccont = 2.1 g L−1 | Formation: Heavy polymer species are adsorbed on the catalytic surface along reaction, leading to the blockage of the redox cycle by limiting the reoxidation of the catalytic sites. The increase in the number of catalytic oxidation sites increased the accumulation of adsorbed species, while high metal dispersion allowed their degradation. Characterization: N/A Regeneration: N/A | [11] |
| CWAO | Cu-PhB-CNF-K | Phenol Industrial wastewater | T = 180–230 °C PO2 = 12–16 bar Ccat = 0.25–1 g L−1 Ccont (phenol) = 1–1.5 g L−1 COD = 111–120 gO2 L−1 | Formation: Carbonaceous deposits formation due to copolymerization of phenol, leading to coverage of catalytic active sites and the deactivation of the catalyst. Characterization: N/A Regeneration: N/A | [12] |
| CWPO | Dissolved iron (Fe3+) | 4-chlorophenol | T = 50 °C pH0 = 3 Ccat = 1–20 mg L−1 CH2O2 = 20–100% stoich. dose Ccont = 0.1–2 g L−1 | Formation: At short reaction times and/or using substoichiometric doses of H2O2, the development of a brownish color in the reaction medium and the existence of a dark solid residue after evaporation were observed. Final colorless effluents were obtained when the stoichiometric dose of H2O2 was used. Characterization: GC-MS of homogeneous condensation by-products and elemental analysis of the solid. Different dimers such as chlorinated diphenyl ethers, PCBs and even dioxins were identified in the liquid phase. The solid mainly contained carbon, oxygen and chlorine, including also iron (46.4% C, 2.6% H, 12.6% Cl, 3.6% Fe and 34.8% O). Regeneration: N/A | [5] |
| CWPO | Fe/pillared beidellite | Phenol | T = 50 °C pH0 = 3.5 Ccat =0.5 g L−1 CH2O2 = 1.25 g L−1 (stoich. dose) Ccont = 0.25 g L−1 | Formation: Organic species were deposited in the used catalyst, leading to a loss of activity upon successive runs. It recovered its initial activity after a calcination step. Characterization: N/A Regeneration: Calcination. | [13] |
| CWPO | Fe2O3/γ-Al2O3 | 2-chlorophenol 2,4-dichlorophenol 2,4,6-trichlorophenol | T = 50 °C pH0 = 3 Ccat = 2 g L−1 CH2O2 = 20–100% stoich. dose Ccont = 0.1–2 g L−1 | Formation: Condensation by-products were formed in high amounts at initial reaction times and/or substoichiometric doses of H2O2. A dramatic color change of the reaction medium was observed. The used catalysts showed a brown color when doses below 75% of H2O2 were used. Characterization: Specific surface area and elemental analysis. The former decreased due to the presence of carbonaceous deposits at H2O2 doses below 75% of the stoichiometric amount (up to 33% area reduction with a 20% H2O2 stoichiometric dose). The used catalyst with 20% H2O2 stoichiometric dose showed 7.7% C, 0.99% H and 2.74% Cl. Regeneration: N/A | [7] |
| CWPO | Activated carbon | Phenol | T = 80 °C pH0 = 3.5 Ccat = 2.5 g L−1 CH2O2 = 100% stoichiometric dose Ccont = 5 g L−1 | Formation: Condensation by-products were adsorbed onto the carbon surface, causing a progressive deactivation upon successive uses. Characterization: TGA and cyclic voltammetry. The burn-off of condensation products occurs at 350 °C. An important decrease of the electrochemical properties of the catalyst due to the presence of carbon deposits was observed. Regeneration: calcination (350 °C, 24 h). | [3] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintanilla, A.; Diaz de Tuesta, J.L.; Figueruelo, C.; Munoz, M.; Casas, J.A. Condensation By-Products in Wet Peroxide Oxidation: Fouling or Catalytic Promotion? Part II: Activity, Nature and Stability. Catalysts 2019, 9, 518. https://doi.org/10.3390/catal9060518
Quintanilla A, Diaz de Tuesta JL, Figueruelo C, Munoz M, Casas JA. Condensation By-Products in Wet Peroxide Oxidation: Fouling or Catalytic Promotion? Part II: Activity, Nature and Stability. Catalysts. 2019; 9(6):518. https://doi.org/10.3390/catal9060518
Chicago/Turabian StyleQuintanilla, Asunción, Jose L. Diaz de Tuesta, Cristina Figueruelo, Macarena Munoz, and Jose A. Casas. 2019. "Condensation By-Products in Wet Peroxide Oxidation: Fouling or Catalytic Promotion? Part II: Activity, Nature and Stability" Catalysts 9, no. 6: 518. https://doi.org/10.3390/catal9060518
APA StyleQuintanilla, A., Diaz de Tuesta, J. L., Figueruelo, C., Munoz, M., & Casas, J. A. (2019). Condensation By-Products in Wet Peroxide Oxidation: Fouling or Catalytic Promotion? Part II: Activity, Nature and Stability. Catalysts, 9(6), 518. https://doi.org/10.3390/catal9060518