Halogen Bonds Fabricate 2D Molecular Self-Assembled Nanostructures by Scanning Tunneling Microscopy
Abstract
:1. Introduction
1.1. The Definition of Halogen Bonds
1.2. The History Perspective of XB
1.3. The Investigation and Application of XB Focus on Crystal Engineering
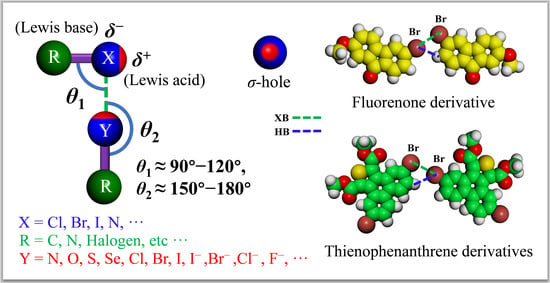
1.4. The General Description of XB in 2D Crystal Engineering
2. Halogen–Halogen Interactions and Halogen-Bonding in 2D Self-Assembled Networks
2.1. The Effect of Geometry Symmetry of π-Conjugated Cores Along the C−Br Bond
2.2. Bifunctional Effect of Benzene Derivative
2.3. Self-Assembled Patterns of Coumarin Derivatives at the 1-Phenyloctane/HOPG Interface
2.4. Self-Assembled Patterns of Thiadiazole Derivatives at the Liquid/HOPG Interface
2.5. Self-Assembled Patterns of Dithiophene Derivative at the Liquid/HOPG Interface
2.6. Self-Assembled Patterns of Fluorenone Derivatives at the Liquid/HOPG Interface
2.7. Self-Assembled Patterns of Phenanthrene Derivatives at the Liquid/HOPG Interface
2.8. Self-Assembled Patterns of Phenanthridine Derivatives at the Liquid/HOPG Interface
2.9. Self-Assembled Patterns of Thienophenanthrene Derivatives at the Liquid/HOPG Interface
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the Halogen Bond. Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding and other σ-hole interactions: A perspective. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Colin, M.M.; Gaultier de Claubry, H. Sur Le Combinaisons de L’iode Avec Les Substances Végétales et Animales. Ann. Chim. 1814, 90, 87–100. [Google Scholar]
- Guthrie, F. Xxviii.—On the Iodide of Iodammonium. J. Chem. Soc. 1863, 16, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Remsen, I.; Norris, J.F. Action of the Halogens on the Methylamines. Am. Chem. J. 1896, 18, 90–95. [Google Scholar]
- Ault, B.S.; Andrews, L. Infrared and Raman spectra of the M + F3− ion pairs and their mixed chlorine-fluorine counterparts in solid argon. Inorg. Chem. 1977, 8, 2024–2028. [Google Scholar] [CrossRef]
- Ault, B.S.; Andrews, L. Matrix reactions of alkali metal fluoride molecules with fluorine. infrared and raman spectra of the trifluoride ion in the M + F3− species. J. Am. Chem. Soc. 1976, 98, 1591–1593. [Google Scholar] [CrossRef]
- Riedel, S.; Köchner, T.; Andrews, P.L.; Wang, X. Polyfluoride Anions, a Matrix-Isolation and Quantum-Chemical Investigation. Inorg. Chem. 2010, 41, 7156–7164. [Google Scholar] [CrossRef]
- Legon, A.C. Prereactive complexes of dihalogens xy with lewis bases b in the gas phase: A systematic case for the halogen analogue b small middle dot small middle dot small middle dotxy of the hydrogen bond b small middle dot small middle dot small middle dotHX. Angew. Chem. Int. Ed. 1999, 38, 2686–2714. [Google Scholar] [CrossRef]
- Brinck, T.; Jane, S.; Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quantum Chem. 1992, 44, 57–64. [Google Scholar] [CrossRef]
- Brinck, T.; Murray, J.S.; Politzer, P. Molecular surface electrostatic potentials and local ionization energies of group v–vii hydrides and their anions: Relationships for aqueous and gas-phase acidities. Int. J. Quantum Chem. 1993, 48, 73–88. [Google Scholar] [CrossRef]
- Murray, J.S.; Paulsen, K.; Politzer, P. Molecular surface electrostatic potentials in the analysis of non-hydrogen-Bonding noncovalent Interactions. Proc. Indian Acad. Sci. Chem. Sci. 1994, 106, 267–275. [Google Scholar]
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.M.; Yamaura, J.I.; Kato, R. Structural and electrical properties of (BEDT-TTF)2X(diiodoacetylene) (X = Cl, Br): The novel self-assembly of neutral Lewis-acidic molecules and halide anions in a molecular metal. J. Mater. Chem. 1998, 8, 15–16. [Google Scholar] [CrossRef]
- Maginn, S.J. Crystal engineering: The design of organic solids by G. R. Desiraju. J. Appl. Crystallogr. 1991, 24, 265. [Google Scholar] [CrossRef] [Green Version]
- Desiraju, G.R. Supramolecular synthons in crystal engineering-a new organic synthesis. Angew. Chem. Int. Ed. 2010, 34, 2311–2327. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From molecules to crystal engineering: Supramolecular isomerism and polymorphism in network solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Zaworotko, M.J. Exploitation of the hydrogen bond: Recent developments in the context of crystal engineering. Coord. Chem. Rev. 1994, 137, 357–401. [Google Scholar] [CrossRef]
- Atwood, J.L.; Lehn, J.M. Comprehensive supramolecular chemistry. solid-state supramolecular chemistry. Cryst. Eng. 1996, 6, 199–206. [Google Scholar]
- Tabellion, F.M.; Seidel, S.R.; Arif, A.M.; Stang, P.J. Discrete supramolecular architecture vs. crystal engineering: The rational design of a platinum-based bimetallic assembly with a chair like structure and its infinite, copper analogue. J. Am. Chem. Soc. 2001, 123, 7740–7741. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Fan, X.; Locklin, J.; Advincula, R.C.; Gies, A.; Nonidez, W. Characterization, supramolecular assembly, and nanostructures of thiophene dendrimers. J. Am. Chem. Soc. 2004, 126, 8735–8743. [Google Scholar] [CrossRef] [PubMed]
- Cook, T.R.; Zheng, Y.R.; Stang, P.J. Metal–organic frameworks and self-assembled supramolecular coordination complexes: Comparing and contrasting the design, synthesis, and functionality of metal–organic materials. Chem. Rev. 2013, 113, 734–777. [Google Scholar] [CrossRef] [Green Version]
- Li, H.L.; Eddaoudi, M.M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- Kovbasyuk, L.; Krämer, R. Allosteric supramolecular receptors and catalysts. Chem. Rev. 2004, 104, 3161–3187. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042–6065. [Google Scholar] [CrossRef]
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821–836. [Google Scholar] [CrossRef] [Green Version]
- Buerkle, L.E.; Rowan, S.J. Supramolecular gels formed from multi-component low molecular weight species. Chem. Soc. Rev. 2012, 41, 6089–6102. [Google Scholar] [CrossRef]
- Aida, T.; Meijer, E.W.; Stupp, S.I. Functional supramolecular polymers. Science 2012, 335, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Stupp, S.I.; Hartgerink, J.D.; Beniash, E. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar]
- Gui, S.; Huang, Y.; Zhu, Y.; Jin, Y.; Rui, Z. Biomimetic sensing system for tracing pb2+ distribution in living cells based on the metal-peptide supramolecular assembly. ACS Appl. Mater. Interfaces 2019, 11, 5804–5811. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.B.; Ferris, C.; Orf, G.; Kavadiya, S.; Ray, J.R.; Jun, Y.S.; Lee, B.; Blankenship, R.E.; Biswas, P. Supramolecular self-assembly of bacteriochlorophyll c molecules in aerosolized droplets to synthesize biomimetic chlorosomes. J. Photochem. Photobiol. B 2018, 185, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Sanjeeva, K.B.; Pigliacelli, C.; Gazzera, L.; Dichiarante, V.; Bombelli, F.B.; Metrangolo, P. Halogen bond-assisted self-assembly of gold nanoparticles in solution and on a planar surface. Nanoscale 2019, 11, 18407–18415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirman, T.; Arad, T.; van der Boom, M.E. Halogen bonding: A supramolecular entry for assembling nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Bent, H.A. Structural chemistry of donor-acceptor interactions. Chem. Rev. 1968, 68, 587–648. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Desiraju, G.R. Crystal engineering and organometallic architecture. Chem. Rev. 1998, 98, 1375–1406. [Google Scholar] [CrossRef]
- Aakery, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2009, 12, 22–43. [Google Scholar] [CrossRef]
- Cannon, A.S.; Warner, J.C. Noncovalent derivatization: Green chemistry applications of crystal engineering. Cryst. Growth Des. 2002, 2, 255–257. [Google Scholar] [CrossRef]
- Teyssandier, J.; Mali, K.S.; De Feyter, S. Halogen bonding in two-dimensional crystal engineering. ChemistryOpen 2020, 9, 225–241. [Google Scholar] [CrossRef]
- Aakery, C.B.; Panikkattu, S.; Chopade, P.D.; Desper, J. Competing hydrogen-bond and halogen-bond donors in crystal engineering. CrystEngComm 2013, 15, 3125–3136. [Google Scholar] [CrossRef] [Green Version]
- Messina, M.T.; Metrangolo, P.; Panzeri, W.; Ragg, E.; Resnati, G. Perfluorocarbon-hydrocarbon self-assembly. part 3. liquid phase interactions between perfluoroalkylhalides and heteroatom containing hydrocarbons. Tetrahedron Lett. 1998, 39, 9069–9072. [Google Scholar] [CrossRef]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 2010, 47, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. Fluorine-centered halogen bonding: A factor in recognition phenomena and reactivity. Cryst. Growth Des. 2011, 11, 4238–4246. [Google Scholar] [CrossRef]
- Metrangolo, P.; Murray, J.S.; Pilati, T.; Politzer, P.; Resnati, G.; Terraneo, G. The fluorine atom as a halogen bond donor, viz. a positive site. CrystEngComm 2011, 13, 6593–6896. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef]
- Mitzel, N.W.; Blake, A.J.; Rankin, D.W.H. Beta-donor bonds in sion units: An inherent structure-determining property leading to (4+4)-coordination in tetrakis-(n,n-dimethylhydroxylamido)silane. J. Am. Chem. Soc. 1997, 119, 4143–4148. [Google Scholar] [CrossRef]
- Hennemann, M.; Murray, J.S.; Politzer, P.; Riley, K.E.; Clark, T. Polarization-induced σ-holes and hydrogen bonding. J. Mol. Model. 2012, 18, 2461–2469. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. A unified view of halogen bonding, hydrogen bonding and other σ-hole interactions. In Noncovalent Forces; Scheiner, S., Ed.; Springer: Cham, Switzerland, 2015; Volume 19, pp. 291–321. [Google Scholar]
- Murray, J.S.; Politzer, P. Hydrogen bonding: A coulombic σ-hole interaction. J. Indian Inst. Sci. 2020, 100, 21–30. [Google Scholar] [CrossRef]
- Sakurai, T.; Sundaralingam, M.; Jeffrey, G.A. A nuclear quadrupole resonance and X-ray study of the crystal structure of 2,5-dichloroaniline. Acta Crystallogr. 2010, 16, 354–363. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Parthasarathy, R. The nature of halogen···halogen interactions: Are short halogen contacts due to specific attractive forces or due to close packing of non-spherical atoms? J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Silly, F.; Viala, C.; Bonvoisin, J. Two-dimensional halogen-bonded porous self-assembled nanoarchitectures of copper β-diketonato complexes. J. Phys. Chem. C 2018, 122, 17143–17148. [Google Scholar] [CrossRef]
- Chang, M.H.; Jang, W.J.; Lee, M.W.; Jeon, U.S.; Han, S.; Kahng, S.J. Networks of non-planar molecules with halogen bonds studied using scanning tunneling microscopy on Au (111). Appl. Surf. Sci. 2018, 432, 110–114. [Google Scholar] [CrossRef]
- Silly, F.; Shaw, A.Q.; Castell, M.R.; Briggs, G.A.D. A chiral pinwheel supramolecular network driven by the assembly of ptcdi and melamine. Chem. Commun. 2008, 1907–1909. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, S.; Hisaki, I.; Nakagawa, S.; Ikenaka, N.; Deng, K.; Xiao, X.; Zeng, Q.D. On-surface self-assembly of a c 3-symmetric π-conjugated molecule family studied by STM: Two-dimensional nanoporous frameworks. Chem. Asian J. 2017, 5, 2558–2564. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Chen, W.; Zeng, Q.; Bo, X.; Bai, C. Alkane-assisted adsorption and assembly of phthalocyanines and porphyrins. J. Am. Chem. Soc. 2000, 122, 5550–5556. [Google Scholar] [CrossRef]
- Silly, F. Selecting two-dimensional halogen-halogen bonded self-assembled 1,3,5-tris(4-iodophenyl)benzene porous nanoarchitectures at the solid–liquid interface. J. Phys. Chem. C 2013, 117, 20244–20249. [Google Scholar] [CrossRef]
- Peyrot, D.; Silly, F. On-surface synthesis of two-dimensional covalent organic structures versus halogen-bonded self-assembly: Competing formation of organic nanoarchitectures. ACS Nano 2016, 10, 5490–5498. [Google Scholar] [CrossRef]
- Gatti, R.; Macleod, J.M.; Lipton-Duffin, J.A.; Moiseev, A.G.; Perepichka, D.F.; Rosei, F. Substrate, Molecular structure, and solvent effects in 2d self-assembly via hydrogen and halogen bonding. J. Phys. Chem. C 2014, 118, 25505–25516. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bond: A long overlooked interaction. In Halogen Bonding I. Topics in Current Chemistry; Metrangolo, P., Resnati, G., Eds.; Springer: Cham, Switzerland, 2014; Volume 358, pp. 1–17. [Google Scholar]
- Binnig, G.; Rohrer, H. The scanning tunneling microscope. Sci. Am. 1985, 253, 50–56. [Google Scholar] [CrossRef]
- Park, S.I.; Quate, C.F. Scanning tunneling microscope. Rev. Sci. Instrum. 1987, 58, 2010–2017. [Google Scholar] [CrossRef]
- Goronzy, D.P.; Ebrahimi, M.; Rosei, F.; Arramel; Fang, Y.; De Feyter, S.; Tait, S.L.; Perepichka, D.F. Supramolecular assemblies on surfaces: Nanopatterning, functionality, and reactivity. ACS Nano 2018, 12, 7445–7481. [Google Scholar] [CrossRef] [PubMed]
- Mate, E. Scientific Conferences: A big hello to halogen bonding. Nat. Chem. 2014, 6, 762–764. [Google Scholar]
- Xing, L.; Jiang, W.; Huang, Z.; Liu, J.; Song, H.; Zhao, W.; Dai, J.; Zhu, H.; Wang, Z.; Weiss, P.S. Steering two-dimensional porous networks with σ-hole interactions of Br···S and Br···Br. Chem. Mater. 2019, 31, 3041–3048. [Google Scholar] [CrossRef]
- Mukherjee, A.; Teyssandier, J.; Hennrich, G.; De Feyter, S.; Mali, K.S. Two-dimensional crystal engineering using halogen and hydrogen bonds: Towards structural landscapes. Chem. Sci. 2017, 8, 3759–3769. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.N.; Liu, X.H.; Chen, T.; Yan, H.J.; Cook, T. Formation of halogen bond-based 2d supramolecular assemblies by electric manipulation. J. Am. Chem. Soc. 2015, 137, 6128–6131. [Google Scholar] [CrossRef]
- Yasuda, S.; Furuya, A.; Murakoshi, K. Control of a two-dimensional molecular structure by cooperative halogen and hydrogen bonds. RSC Adv. 2014, 4, 58567–58572. [Google Scholar] [CrossRef] [Green Version]
- Gutzler, R.; Fu, C.; Dadvand, A.; Hua, Y.; Macleod, J.M.; Rosei, F.; Perepichka, D.F. Halogen Bonds in 2d Supramolecular self-assembly of organic semiconductors. Nanoscale 2012, 4, 5965–5971. [Google Scholar] [CrossRef]
- Li, J.X.; Wu, J.T.; Chen, S.W.; Miao, X.R.; Fabien, S.; Deng, W.L. Geometry symmetry of conjugated cores along C–Br bond effect on the 2d self-assembly by intermolecular H···Br and Br···Br Bonds. J. Phys. Chem. C 2018, 433, 1075–1082. [Google Scholar] [CrossRef]
- Blanco, M.A.; Martín, P.A.; Francisco, E. Interacting quantum atoms: A correlated energy decomposition scheme based on the quantum theory of atoms in molecules. J. Chem. Theory Comput. 2005, 1, 1096–1109. [Google Scholar] [CrossRef]
- Popelier, P.L.A. Molecular similarity and complementarity based on the theory of atoms in molecules. In Molecular Similarity in Drug Design; Dean, P.M., Ed.; Springer: Dordrecht, The Netherlands, 1995; Volume 358, pp. 215–240. [Google Scholar]
- Wu, Y.C.; Li, J.X.; Yuan, Y.; Dong, M.Q.; Zha, B.; Miao, X.R.; Hu, Y.; Deng, W.L. Halogen bonding versus hydrogen bonding induced 2d self-assembled nanostructures at the liquid-solid interface revealed by STM. Phys. Chem. Chem. Phys. 2016, 19, 3143–3150. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, L.; Subramani, R.; Pan, Y.; Bo, L.; Yang, Y.; Chen, W.; Mamdouh, W.; Besenbacher, F.; Dong, M. Building layer-by-layer 3d supramolecular nanostructures at the terephthalic acid/stearic acid interface. Chem. Commun. 2011, 47, 9155–9157. [Google Scholar] [CrossRef] [PubMed]
- Nagula, R.G.; Bolton, O.; Burgess, E.C.; Matzger, A.J. The unprecedented size of the σ-holes on 1,3,5-triiodo-2,4,6-trinitrobenzene begets unprecedented intermolecular interactions. Cryst. Growth Des. 2016, 16, 1765–1771. [Google Scholar]
- Wu, J.T.; Li, J.X.; Dong, M.Q.; Miao, K.; Miao, X.R.; Wu, Y.C.; Deng, W.L. Solvent effect on host-guest two-dimensional self-assembly mediated by halogen bonding. J. Phys. Chem. C 2018, 122, 22597–22604. [Google Scholar] [CrossRef]
- Zha, B.; Li, J.X.; Wu, J.T.; Miao, X.R.; Zhang, M. Cooperation and competition of hydrogen and halogen bonds in 2d self-assembled nanostructures based on bromine substituted coumarins. New J. Chem. 2019, 43, 17182–17187. [Google Scholar] [CrossRef]
- Zha, B.; Miao, X.R.; Li, Y.; Liu, P.; Deng, W.L. Solvent-dependent self-assembly of 4,7-dibromo-5,6-bis(octyloxy)benzo[c][1,2,5] thiadiazole on graphite surface by scanning tunneling microscopy. J. Nanomater. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Hu, T.Z.; Wang, Y.J.; Dong, M.Q.; Wu, J.T.; Pang, P.; Miao, X.R.; Deng, W.L. Ordering self-assembly structures via intermolecular Brs interactions. Phys. Chem. Chem. Phys. 2020, 22, 1437–1443. [Google Scholar] [CrossRef]
- Dong, M.Q.; Miao, K.; Wu, J.T.; Miao, X.R.; Li, J.X.; Pang, P.; Deng, W.L. Halogen substituent effects on concentration-controlled self-assembly of fluorenone derivatives: Halogen bond versus hydrogen bond. J. Phys. Chem. C 2019, 123, 4349–4359. [Google Scholar] [CrossRef]
- Dong, M.Q.; Wu, J.T.; Miao, X.R.; Li, J.X.; Deng, W.L. Bromine substituent position triggered halogen versus hydrogen bond in 2d self-assembly of fluorenone derivatives. J. Phys. Chem. C 2019, 123, 26191–26200. [Google Scholar] [CrossRef]
- Dong, M.Q.; Hu, T.Z.; Wang, Y.; Pang, P.; Wang, Y.J.; Miao, X.R.; Deng, W.L. Halogen-bonded building block for 2D self-assembly: Triggered by hydrogen-bonding motifs relative to the terminal functions of the side chains. Appl. Surf. Sci. 2020, 515, 145983–145992. [Google Scholar] [CrossRef]
- Hu, X.Y.; Zha, B.; Wu, Y.C.; Miao, X.R.; Deng, W.L. Effects of the position and number of bromine substituents on the concentration-mediated 2d self-assembly of phenanthrene derivatives. Phys. Chem. Chem. Phys. 2016, 18, 7208–7215. [Google Scholar] [CrossRef] [PubMed]
- Pang, P.; Miao, X.R.; Ying, L.; Kong, G.; Deng, W.L. Halogen-bond-controlled self-assembly of regioisomeric phenanthridine deriatives into nanowires and nanosheets. J. Phys. Chem. C 2020, 124, 5665–5671. [Google Scholar] [CrossRef]
- Zha, B.; Dong, M.Q.; Miao, X.R.; Peng, S.; Wu, Y.C.; Miao, K.; Hu, Y.; Deng, W.L. Cooperation and competition between halogen bonding and van der waals forces in supramolecular engineering at the aliphatic hydrocarbon/graphite interface: Position and number of bromine group effects. Nanoscale 2016, 9, 237–250. [Google Scholar] [CrossRef] [PubMed]
- Zha, B.; Miao, X.R.; Liu, P.; Wu, Y.C.; Deng, W.L. Concentration dependent halogen-bond density in the 2d self-assembly of a thienophenanthrene derivative at the aliphatic acid/graphite interface. Chem. Commun. 2014, 50, 9003–9006. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Luo, Y.; Zhang, Y.; Yu, Y.J.; Kuang, Y.M.; Zhang, L.; Meng, Q.S.; Luo, Y.; Yang, J.L.; Dong, Z.C. Visualizing coherent intermolecular dipole–dipole coupling in real space. Nature 2016, 531, 623–627. [Google Scholar] [CrossRef]
- Zha, B.; Dong, M.Q.; Miao, X.; Miao, K.; Hu, Y.; Wu, Y.C.; Xu, L.; Deng, W.L. Controllable orientation of ester-group-induced intermolecular halogen bonding in a 2d self-assembly. J. Phys. Chem. Lett. 2016, 7, 3164–3170. [Google Scholar] [CrossRef]
- Gutzler, R.; Ivasenko, O.; Fu, C.; Brusso, J.L.; Rosei, F.; Perepichka, D.F. Halogen bonds as stabilizing interactions in a chiral self-assembled molecular monolayer. Chem. Commun. 2011, 47, 9453–9455. [Google Scholar] [CrossRef]
- Miao, X.R.; Li, J.X.; Zha, B.; Miao, K.; Dong, M.Q.; Wu, J.T.; Deng, W.L. Concentration-dependent multiple chirality transition in halogen-bond-driven 2d self-assembly process. Appl. Surf. Sci. 2018, 433, 1075–1082. [Google Scholar] [CrossRef]
- Wu, J.T.; Li, J.X.; Miao, X.R.; Ying, L.; Dong, M.Q.; Deng, W.L. The brmidline horizontal ellipsis π-halogen bond assisted self-assembly of an asymmetric molecule regulated by concentration. Chem. Commun. 2020, 56, 2727–2730. [Google Scholar] [CrossRef]


















Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Miao, X.; Deng, W. Halogen Bonds Fabricate 2D Molecular Self-Assembled Nanostructures by Scanning Tunneling Microscopy. Crystals 2020, 10, 1057. https://doi.org/10.3390/cryst10111057
Wang Y, Miao X, Deng W. Halogen Bonds Fabricate 2D Molecular Self-Assembled Nanostructures by Scanning Tunneling Microscopy. Crystals. 2020; 10(11):1057. https://doi.org/10.3390/cryst10111057
Chicago/Turabian StyleWang, Yi, Xinrui Miao, and Wenli Deng. 2020. "Halogen Bonds Fabricate 2D Molecular Self-Assembled Nanostructures by Scanning Tunneling Microscopy" Crystals 10, no. 11: 1057. https://doi.org/10.3390/cryst10111057
APA StyleWang, Y., Miao, X., & Deng, W. (2020). Halogen Bonds Fabricate 2D Molecular Self-Assembled Nanostructures by Scanning Tunneling Microscopy. Crystals, 10(11), 1057. https://doi.org/10.3390/cryst10111057