Calcination of Calcium Sulphoaluminate Cement Using Pyrite-Rich Cyanide Tailings
Abstract
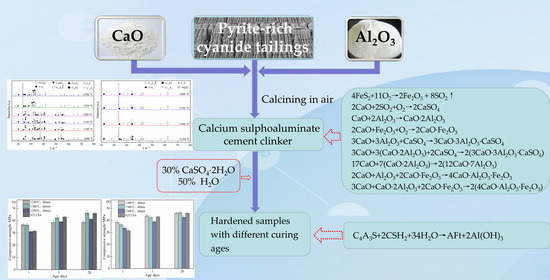
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Experimental Procedure
2.3. Analysis
2.4. Calculation
- mm is the mass of the mixture;
- mc is the mass of the cement clinker;
- wc/m is the mass ratio of the cement clinker to the mixture;
- wm is the mass ratio of S in the mixture;
- wc is the mass ratio of S in the cement clinker.
3. Results and Discussion
3.1. Characterization of the Raw Materials
3.2. Compressive Strength of the Cement Clinker
3.3. Behavior of Pyrite in CTs During Calcination
3.4. Preparation of the Cement Clinker
3.4.1. Effect of Calcination Temperature
3.4.2. Effect of Calcination Time
3.5. Mineral Phases of the Cement Clinker
3.5.1. XRD Patterns at Different Calcination Temperatures
3.5.2. XRD Patterns at Different Calcination Times
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Habert, G. Assessing the environmental impact of conventional and ‘green’ cement production. In Eco-Efficient Construction and Building Materials; Elsevie: Amsterdam, The Netherlands, 2014; pp. 199–238. [Google Scholar]
- Wang, Y.; Su, M.; Zhang, L. Sulphoaluminate Cement; Beijing University of Technology Press: Beijing, China, 1999. [Google Scholar]
- Bullerjahn, F.; Schmitt, D.; Ben Haha, M. Effect of raw mix design and of clinkering process on the formation and mineralogical composition of (ternesite) belite calcium sulphoaluminate ferrite clinker. Cem. Concr. Res. 2014, 59, 87–95. [Google Scholar] [CrossRef]
- Wu, S.; Wang, W.; Ren, C.; Yao, X.; Yao, Y.; Zhang, Q.; Li, Z. Calcination of calcium sulphoaluminate cement using flue gas desulfurization gypsum as whole calcium oxide source. Constr. Build. Mater. 2019, 228, 116676. [Google Scholar] [CrossRef]
- Shen, Y.; Qian, J.; Chai, J.; Fan, Y. Calcium sulphoaluminate cements made with phosphogypsum: Production issues and material properties. Cem. Concr. Compos. 2014, 48, 67–74. [Google Scholar] [CrossRef]
- Xue, P.; Xu, A.; He, D.; Yang, Q.; Liu, G.; Engström, F.; Björkman, B. Research on the sintering process and characteristics of belite sulphoaluminate cement produced by BOF slag. Constr. Build. Mater. 2016, 122, 567–576. [Google Scholar] [CrossRef]
- Zhou, H.; Liu, J.; Liu, J.; Li, C. Hydration kinetics process of low alkalinity sulphoaluminate cement and its thermodynamical properties. Procedia Eng. 2012, 27, 323–331. [Google Scholar] [CrossRef] [Green Version]
- Schneider, M.; Romer, M.; Tschudin, M.; Bolio, H. Sustainable cement production—present and future. Cem. Concr. Res. 2011, 41, 642–650. [Google Scholar] [CrossRef]
- Mao, Y.; Wu, H.; Wang, W.; Jia, M.; Che, X. Pretreatment of municipal solid waste incineration fly ash and preparation of solid waste source sulphoaluminate cementitious material. J. Hazard. Mater. 2020, 385, 121580. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, X.; Zhang, W.; Li, X.; Qian, J. Influence of ternesite on the properties of calcium sulfoaluminate cements blended with fly ash. Constr. Build. Mater. 2018, 193, 221–229. [Google Scholar] [CrossRef]
- Jin, Z.; Ma, B.; Su, Y.; Lu, W.; Qi, H.; Hu, P. Effect of calcium sulphoaluminate cement on mechanical strength and waterproof properties of beta-hemihydrate phosphogypsum. Constr. Build. Mater. 2020, 242, 118198. [Google Scholar] [CrossRef]
- Gao, D.; Meng, Y.; Yang, L.; Tang, J.; Lv, M. Effect of ground granulated blast furnace slag on the properties of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 227, 116665. [Google Scholar] [CrossRef]
- Ge, Z.; Yuan, H.; Sun, R.; Zhang, H.; Wang, W.; Qi, H. Use of green calcium sulphoaluminate cement to prepare foamed concrete for road embankment: A feasibility study. Constr. Build. Mater. 2020, 237, 117791. [Google Scholar] [CrossRef]
- Da Costa, E.B.; Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Gobbo, L.A.; Kirchheim, A.P. Production and hydration of calcium sulfoaluminate-belite cements derived from aluminium anodising sludge. Constr. Build. Mater. 2016, 122, 373–383. [Google Scholar] [CrossRef]
- Lv, C.; Ding, J.; Qian, P.; Li, Q.; Ye, S.; Chen, Y. Comprehensive recovery of metals from cyanidation tailing. Miner. Eng. 2015, 70, 141–147. [Google Scholar] [CrossRef]
- Lei, Z.; Guangfeng, K.; Shufen, L.; Xianfeng, C.; Xianyang, W. Research on multi-element resources of utilizing cyaniding tailings. Environ. Sci. Technol. 2010, 23, 5–7. [Google Scholar]
- Order no. 61 of the President of the People’s Republic of China. Environmental Protection Tax Law of the People’s Republic of China; Republic of China: Beijing, China, 2016; Chapter V.
- Barcelos, D.A.; Pontes, F.V.; Da Silva, F.A.; Castro, D.C.; Dos Anjos, N.O.; Castilhos, Z.C. Gold mining tailing: Environmental availability of metals and human health risk assessment. J. Hazard. Mater. 2020, 397, 122721. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, C. Applying alkali-chlorination method in treatment for cyanide containing gold tailing sands. Environ. Sanit. Eng. 2009, 17, 42–44. [Google Scholar]
- Xing, W.; Jin, Y.; Yao, X. Discussion on some issues in dry stack technology of tailings from gold mines. Nonferr. Metals (Min.) 2008, 60, 48–50. [Google Scholar]
- Qian-jin, G. Discussion on treatment technology of cyanide waste in sodium cyanide plant. J. Shanxi Coal-Min. Adm. Col. 2004, 87–88. [Google Scholar]
- Khamar, Z.; Makhdoumi-Kakhki, A.; Gharaie, M.M. Remediation of cyanide from the gold mine tailing pond by a novel bacterial co-culture. Int. Biodeterior. Biodegrad. 2015, 99, 123–128. [Google Scholar] [CrossRef]
- Ritcey, G.M. Tailings management in gold plants. Hydrometallurgy 2005, 78, 3–20. [Google Scholar] [CrossRef]
- Amaratunga, L.M.; Hmidi, N. Cold-bond agglomeration of gold mill tailings for backfill using gypsum betahemihydrate and cement as low cost binders. Can. Metal. Quart. 1997, 36, 283–288. [Google Scholar] [CrossRef]
- Jiao, X.; Liu, X. Restoring tailing pool of gold mine to farmland. Gold 2004, 25, 46–47. [Google Scholar]
- GB 18598-2001. Standard for Pollution Control on the Security Landfill Site for Hazardous Wastes; China Standard Press: Beijing, China, 2001; pp. 421–429. [Google Scholar]
- Li, H.; Long, H.; Zhang, L.; Yin, S.; Li, S.; Zhu, F.; Xie, H. Effectiveness of microwave-assisted thermal treatment in the extraction of gold in cyanide tailings. J. Hazard. Mater. 2020, 384, 121456. [Google Scholar] [CrossRef]
- Long, H.; Ma, A.; Srinivasakannan, C.; Zhang, L.; Li, S.; Yin, S. Investigation on the recovery of gold and silver from cyanide tailings using chlorination roasting process. J. Alloy. Compd. 2018, 763, 241–249. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Li, H.-M.; Yu, X.-J. Fe extraction from high-silicon and aluminum cyanide tailings by pretreatment of water leaching before magnetic separation. Trans. Nonferrous Met. Soc. China 2013, 23, 1165–1173. [Google Scholar] [CrossRef]
- Yang, X.; Huang, X.; Qiu, T. Recovery of zinc from cyanide tailings by flotation. Miner. Eng. 2015, 84, 100–105. [Google Scholar] [CrossRef]
- Zhang, M.; Cao, Y.; Peng, B.; Tian, Y.; Barvor, J.B. Removal of copper cyanide by precipitate flotation with ammonium salts. Process. Saf. Environ. Prot. 2020, 133, 82–87. [Google Scholar] [CrossRef]
- Junfeng, G.; Xiaobo, L. Utilization of cyanided tailings from gold ore dressing plant. Miner. Eng. 2005, 3, 38–39. [Google Scholar]
- Roy, S.; Adhikari, G.R.; Gupta, R.N. Use of gold mill tailings in making bricks: A feasibility study. Waste Manag. Res. 2007, 25, 475–482. [Google Scholar] [CrossRef]
- Shao, H.; Liang, K.; Peng, F.; Zhou, F.; Hu, A. Production and properties of cordierite-based glass-ceramics from gold tailings. Miner. Eng. 2005, 18, 635–637. [Google Scholar] [CrossRef]
- Ding, Y.; Wu, W. Preparation of aerated concrete block by making use of the gold tailings. New Build. Mater. 2009, 38–40. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhao, Y. Utilization of metal mine solid waste in production of construction materials. Nonferr. Metal Eng. Res. 2008, 29, 35–38. [Google Scholar]
- Zhang, S.; Shuai, G. Charge calculation of sulfoaluminate belite cement clinker. J. Nanchang Univ. (Eng. Technol.) 2011, 33, 30–32. [Google Scholar]
- GB/T 6730. 8-2016. Iron Ores-Determination of Iron (Ⅱ) Content-Potassium Dichromate Titrimetric Method; China Standard Press: Beijing, China, 2017; p. 12. [Google Scholar]
- MEP. China, Soil-Determination of Cyanide and Total Cyanide-Spectrometric Method; China Environmental Science Press: Beijing, China, 2015. [Google Scholar]
- Phutthimethakul, L.; Kumpueng, P.; Supakata, N. Use of Flue Gas Desulfurization Gypsum, Construction and Demolition Waste, and Oil Palm Waste Trunks to Produce Concrete Bricks. Crystals 2020, 10, 709. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, S.; Liu, S.; Wang, L.; Guan, X. Effect of preparation conditions on the shape and distribution of MgO in clinker. J. Wuhan Univ. Technol. 2013, 35, 27–32. [Google Scholar]
- Dong, K.; Xie, F.; Chang, Y.; Chen, C.; Wang, W.; Lu, D.; Gu, X. A novel strategy for the efficient decomposition of toxic sodium cyanate by hematite. Chemosphere 2020, 256, 127047. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, C.; Li, B.; Wang, C.; Chen, X.; Wei, J.; Yu, Q. Clarifying the decomposition process of pyrite and SO2 release in the cyclone preheater of a dry rotary cement kiln system. J. Clean. Prod. 2019, 241, 118422. [Google Scholar] [CrossRef]
- Li, L.; Wang, R.; Zhang, S. Effect of curing temperature and relative humidity on the hydrates and porosity of calcium sulfoaluminate cement. Constr. Build. Mater. 2019, 213, 627–636. [Google Scholar] [CrossRef]
- Zhai, H.; Bian, C.; Yu, Y.; Zhu, L.; Guo, L.; Wang, X.; Yu, Q.; Zhu, J.; Cao, X. Sustainable Route for Synthesis of All-Silica SOD Zeolite. Crystals 2019, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Colville, A.A.; Geller, S. The crystal structure of brownmillerite, Ca2FeAlO5. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 2311–2315. [Google Scholar] [CrossRef]
- Salasin, J.R.; Rawn, C. Structure Property Relationships and Cationic Doping in [Ca24Al28O64]4+ Framework: A Review. Crystals 2017, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Ceretti, M.; Corallini, S.; Paulus, W. Influence of Phase Transformations on Crystal Growth of Stoichiometric Brownmillerite Oxides: Sr2ScGaO5 and Ca2Fe2O5. Crystals 2016, 6, 146. [Google Scholar] [CrossRef] [Green Version]
- Boyko, E.; Wisnyl, L.G. The optical properties and structures of CaO2Al2O3and SrO2Al2O3. Acta Crystallogr. 1958, 11, 444–445. [Google Scholar] [CrossRef]
- Natta, G.; Passerini, L. Soluzioni solide, isomorfismo e simmorfismo tra gli ossidi dei metalli bivalenti. Sistemi: CaO-CdO. CaOMnO, CaO-CoO, CaO-NiO, CaO- MgO. Gazz. Chim. Ital. 1929, 59, 129–154. (In Italian) [Google Scholar]
- Schmahl, N.G.; Eikerling, G.F. Über Kryptomodifikationen desCu(II)-Oxids. Z. Phys. Chem. 1968, 62, 268–279. [Google Scholar] [CrossRef]





| Raw Materials | Total Fe | S | SiO2 | CaO | Al2O3 | LOI | Total Cyanide |
|---|---|---|---|---|---|---|---|
| Cyanide tailings | 41.41 | 48.40 | 5.72 | 0.47 | 1.48 | – | 339.14 × 10−6 |
| Calcium carbonate | <0.01 | <0.01 | 0.01 | 55.91 | 0.01 | 43.88 | – |
| Alumina | 0.01 | 0.02 | 0.04 | 0.02 | 94.60 | 5.09 | – |
| Mineral Phases | PDF Code | ICSD Code | References |
|---|---|---|---|
| C4A3 | 85-2210 | 80361 | [45] |
| C4AF | 74-1346 | 27112 | [46] |
| C12A7 | 70-2144 | 6287 | [47] |
| C2F | 71-2264 | 15059 | [48] |
| CA2 | 89-3851 | 44519 | [49] |
| f-CaO | 78-0649 | 61550 | [50] |
| MgO | 78-0430 | 61325 | [51] |
| T/°C | C4AF | C12A7 | C2F | CA2 | MgO | R | |
|---|---|---|---|---|---|---|---|
| 1250 | 61.2 | 7.4 | 9.3 | 1.9 | 5.2 | 14.9 | 8.96 |
| 1300 | 64.1 | 11.0 | 9.4 | – | – | 15.3 | 8.08 |
| 1350 | 65.6 | 12.4 | 7.1 | – | – | 14.9 | 8.14 |
| 1400 | 68.8 | 13.5 | 2.9 | – | – | 14.8 | 9.21 |
| t/min | C4AF | C12A7 | C2F | CA2 | CaO | MgO | R | |
|---|---|---|---|---|---|---|---|---|
| 0 | 56.7 | 7.5 | 9.3 | 3.1 | 4.7 | 3.3 | 15.4 | 8.99 |
| 20 | 62.1 | 7.5 | 9.7 | 2.3 | 3.6 | – | 14.9 | 9.30 |
| 40 | 64.1 | 7.0 | 9.1 | 2.3 | 2.4 | – | 15.1 | 9.09 |
| 60 | 64.1 | 11.0 | 9.4 | – | – | – | 15.3 | 8.08 |
| 80 | 64.4 | 10.9 | 9.3 | – | – | – | 15.3 | 8.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, K.; Xie, F.; Wang, W.; Chang, Y.; Chen, C.; Gu, X. Calcination of Calcium Sulphoaluminate Cement Using Pyrite-Rich Cyanide Tailings. Crystals 2020, 10, 971. https://doi.org/10.3390/cryst10110971
Dong K, Xie F, Wang W, Chang Y, Chen C, Gu X. Calcination of Calcium Sulphoaluminate Cement Using Pyrite-Rich Cyanide Tailings. Crystals. 2020; 10(11):971. https://doi.org/10.3390/cryst10110971
Chicago/Turabian StyleDong, Kaiwei, Feng Xie, Wei Wang, Yongfeng Chang, Chunlin Chen, and Xiaowei Gu. 2020. "Calcination of Calcium Sulphoaluminate Cement Using Pyrite-Rich Cyanide Tailings" Crystals 10, no. 11: 971. https://doi.org/10.3390/cryst10110971
APA StyleDong, K., Xie, F., Wang, W., Chang, Y., Chen, C., & Gu, X. (2020). Calcination of Calcium Sulphoaluminate Cement Using Pyrite-Rich Cyanide Tailings. Crystals, 10(11), 971. https://doi.org/10.3390/cryst10110971