Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 1–3
2.2. X-ray Diffractometry
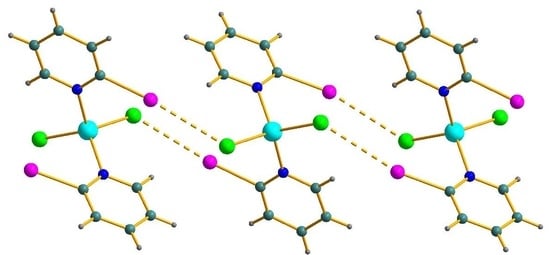
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Sivchik, V.V.; Solomatina, A.I.; Chen, Y.-T.; Karttunen, A.J.; Tunik, S.P.; Chou, P.-T.; Koshevoy, I.O. Halogen bonding to amplify luminescence: A case study using a platinum cyclometalated complex. Angew. Chem. Int. Ed. 2015, 54, 14057–14060. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, B.; Sinha, S.; Ghosh, P. Selective sensing of phosphates by a new bis-heteroleptic RuIIComplex through halogen bonding: A superior sensor over its hydrogen-bonding analogue. Chem. A Eur. J. 2016, 22, 18051–18059. [Google Scholar] [CrossRef]
- Xiao, L.; Wu, Y.; Yu, Z.; Xu, Z.; Li, J.; Liu, Y.; Yao, J.; Fu, H. Room-temperature phosphorescence in pure organic materials: Halogen bonding switching effects. Chem. A Eur. J. 2018, 24, 1801–1805. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Kashina, M.V.; Mikherdov, A.S.; Mozheeva, E.A.; Novikov, A.S.; Smirnov, A.S.; Ivanov, D.M.; Kryukova, M.A.; Ivanov, A.Y.; Smirnov, S.N.; et al. Dramatically enhanced solubility of halide-containing organometallic species in diiodomethane: The role of solvent⋅⋅⋅complex halogen bonding. Angew. Chem. Int. Ed. 2018, 57, 12785–12789. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Samsonenko, D.G.; Yushina, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with halopyridinium cations: Appearance or non-appearance of unusual colouring. CrystEngComm 2018, 20, 7766–7772. [Google Scholar] [CrossRef]
- Priimagi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The halogen bond in the design of functional supramolecular Materials: Recent advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [Green Version]
- Buntara Sanjeeva, K.; Pigliacelli, C.; Gazzera, L.; Dichiarante, V.; Baldelli Bombelli, F.; Metrangolo, P. Halogen bond-assisted self-assembly of gold nanoparticles in solution and on a planar surface. Nanoscale 2019, 11, 18407–18415. [Google Scholar] [CrossRef] [Green Version]
- Saccone, M.; Catalano, L. Halogen bonding beyond crystals in materials science. J. Phys. Chem. B 2019, 123, 9281–9290. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Chen, Y.; Zhao, D.; Lu, X.; Lin, Y. Versatile supramolecular binding modes of 1,4-diiodotetrafluorobenzene for molecular cocrystal engineering. J. Mol. Struct. 2019, 1187, 132–137. [Google Scholar] [CrossRef]
- Saraswatula, V.G.; Saha, B.K. The effect of temperature on interhalogen interactions in a series of isostructural organic systems. New J. Chem. 2014, 38, 897–901. [Google Scholar] [CrossRef]
- Saraswatula, V.G.; Saha, B.K. A thermal expansion investigation of the melting point anomaly in trihalomesitylenes. Chem. Commun. 2015, 51, 9829–9832. [Google Scholar] [CrossRef] [Green Version]
- Buldakov, A.V.; Kinzhalov, M.A.; Kryukova, M.A.; Ivanov, D.M.; Novikov, A.S.; Smirnov, A.S.; Starova, G.L.; Bokach, N.A.; Kukushkin, V.Y. Isomorphous series of PdII-containing halogen bond donors exhibiting Cl/Br/I triple halogen isostructural exchange. Cryst. Growth Des. 2020. [Google Scholar] [CrossRef]
- Chupina, A.V.; Shayapov, V.; Novikov, A.S.; Volchek, V.V.; Benassi, E.; Abramov, P.A.; Sokolov, M.N. [{AgL}2Mo8O26]n− complexes: A combined experimental and theoretical study. Dalt. Trans. 2020, 49, 1522–1530. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Taher, D.; Maabreh, A.; Alwedian, F.Z.; Al-Ebaisat, H.; Rüffer, T.; Lang, H. The role of Fe–X···X–Fe contacts in the crystal structures of [(2-iodopyridinium)2FeX4]X (X = Cl, Br). Struct. Chem. 2013, 24, 401–408. [Google Scholar] [CrossRef]
- Awwadi, F.; Haddad, S.F.; Willett, R.D.; Twamley, B. The Analogy of C−Br···Br−C, C−Br···Br−Fe, and Fe−Br···Br−Fe Contacts: Crystal Structures of (26DAPH)FeBr4 and (26DA35DBPH)2 FeBr4·Br. Cryst. Growth Des. 2010, 10, 158–164. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Taher, D.; Haddad, S.F.; Turnbull, M.M. Competition between hydrogen and halogen bonding interactions: Theoretical and crystallographic studies. Cryst. Growth Des. 2014, 14, 1961–1971. [Google Scholar] [CrossRef]
- Puttreddy, R.; von Essen, C.; Rissanen, K. Halogen bonds in square planar 2,5-dihalopyridine-copper(II) bromide complexes. Eur. J. Inorg. Chem. 2018, 2018, 2393–2398. [Google Scholar] [CrossRef] [Green Version]
- Farris, P.C.; Wall, A.D.; Chellali, J.E.; Chittim, C.L.; Landee, C.P.; Turnbull, M.M.; Wikaira, J.L. Copper(II) halide complexes of aminopyridines: Syntheses, structures and magnetic properties of [(5CAP)2CuX2] and [(5BAP)nCuX2] (X = Cl, Br). J. Coord. Chem. 2018, 71, 2487–2509. [Google Scholar] [CrossRef]
- Abdalrahman, M.; Landee, C.P.; Telfer, S.G.; Turnbull, M.M.; Wikaira, J.L. Copper(II) halide coordination complexes and salts of 3-halo-2-methylpyridines: Synthesis, structure and magnetism. Inorg. Chim. Acta 2012, 389, 66–76. [Google Scholar] [CrossRef]
- Hu, C.; Li, Q.; Englert, U. Structural trends in one and two dimensional coordination polymers of cadmium(ii) with halide bridges and pyridine-type ligands. CrystEngComm 2003, 5, 519. [Google Scholar] [CrossRef]
- Wang, R.; Dols, T.S.; Lehmann, C.W.; Englert, U. The halogen bond made visible: Experimental charge density of a very short intermolecular Cl⋯Cl donor–acceptor contact. Chem. Commun. 2012, 48, 6830. [Google Scholar] [CrossRef] [PubMed]
- Krogul, A.; Cedrowski, J.; Wiktorska, K.; Ozimiński, W.P.; Skupińska, J.; Litwinienko, G. Crystal structure, electronic properties and cytotoxic activity of palladium chloride complexes with monosubstituted pyridines. Dalton Trans. 2012, 41, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Hiltunen, L.; Niinistö, L.; Kenessey, G.; Keserü, G.M.; Liptay, G.; Kondow, A.J.; Bisht, K.S.; Parmar, V.S.; Francis, G.W. Pyridine-Type Complexes of Transition-Metal Halides. V. Preparation, Thermal Properties, Infrared Spectra and Crystal Structure of Dibromo-bis(2-bromopyridine)cobalt(II). Acta Chem. Scand. 1994, 48, 456–460. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willett, R.D.; Twamley, B.; Turnbull, M.M.; Landee, C.P. Dual behavior of bromine atoms in supramolecular chemistry: The crystal structure and magnetic properties of two copper(II) coordination polymers. Cryst. Growth Des. 2015, 15, 3746–3754. [Google Scholar] [CrossRef]
- Awwadi, F.; Willett, R.D.; Twamley, B. Tuning Molecular Structures Using Weak Noncovalent Interactions: Theoretical Study and Structure of trans -Bis(2-chloropyridine)dihalocopper(II) and trans -Bis(3-chloropyridine)dibromocopper(II). Cryst. Growth Des. 2011, 11, 5316–5323. [Google Scholar] [CrossRef]
- Healy, P.C.; Kildea, J.D.; Skelton, B.W.; Waters, A.F.; White, A.H. Lewis-base adducts of group 11 metal(I) compounds. 60. Binuclear adducts of copper(I) halides with 2-hindered pyridine bases. Acta Crystallogr. Sect. C 1991, 47, 1721–1723. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willett, R.D.; Haddad, S.F.; Twamley, B. The electrostatic nature of aryl-bromine-halide synthons: The role of aryl-bromine-halide synthons in the crystal structures of the trans-bis(2-bromopyridine)dihalocopper(II) and trans-bis(3-bromopyridine) dihalocopper(II) complexes. Cryst. Growth Des. 2006, 6, 1833–1838. [Google Scholar] [CrossRef]
- Kenessey, G.; Werner, P.-E.; Wadsten, T.; Bidló, G.; Liptay, G. Pyridine-type complexes of transition-metal Halides VII. Thermal and structural properties of Cobalt(II) Halide complexes formed with 2-Halogenopyridines. Acta Chem. Scand. 1999, 53, 163–167. [Google Scholar] [CrossRef]
- Billing, D.E.; Underhill, A.E. Complexes of cobalt(II) and nickel(II) halides with some halogenopyridines. J. Chem. Soc. A Inorg. Phys. Theor. 1968, 29–33. [Google Scholar] [CrossRef]
- Mortimer, C.T.; McNaughton, J.L. The thermal decomposition of transition-metal complexes containing heterocyclic ligands. 3. Substituted pyridine complexes of cobalt. Thermochim. Acta 1974, 10, 125–128. [Google Scholar] [CrossRef]
- McWhinnie, W.R. The far infra-red spectra of metal complexes containing substituted pyridines as ligands-II Complexes of cobalt (II) and copper (II) with 2-chloro- and 2-bromo-pyridine. J. Inorg. Nucl. Chem. 1965, 27, 2573–2580. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volumes and radii of metals in covalent compounds. J. Phys. Chem. 1966, 70, 3006–3007. [Google Scholar] [CrossRef]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals Radii for the Whole Main Group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [Green Version]
- Zelenkov, L.E.; Ivanov, D.M.; Avdontceva, M.S.; Novikov, A.S.; Bokach, N.A. Tetrachloromethane as halogen bond donor toward metal-bound halides. Z. Krist. Cryst. Mater. 2019, 234, 9–17. [Google Scholar] [CrossRef]
- Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Matveychuk, Y.V.; Bokach, N.A.; Kukushkin, V.Y. Metal-Involving Bifurcated Halogen Bonding C–Br···η2 (Cl–Pt). Cryst. Growth Des. 2019, 19, 1364–1376. [Google Scholar] [CrossRef]
- Burianova, V.K.; Bolotin, D.S.; Mikherdov, A.S.; Novikov, A.S.; Mokolokolo, P.P.; Roodt, A.; Boyarskiy, V.P.; Dar’in, D.; Krasavin, M.; Suslonov, V.V.; et al. Mechanism of generation of closo-decaborato amidrazones. Intramolecular non-covalent B–H···π(Ph) interaction determines stabilization of the configuration around the amidrazone C=N bond. New J. Chem. 2018, 42, 8693–8703. [Google Scholar] [CrossRef]
- Baykov, S.V.; Dabranskaya, U.; Ivanov, D.M.; Novikov, A.S.; Boyarskiy, V.P. Pt/Pd and I/Br isostructural exchange provides formation of C–I···Pd, C–Br···Pt, and C–Br···Pd metal-involving halogen bonding. Cryst. Growth Des. 2018, 18, 5973–5980. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From weak to strong interactions: A comprehensive analysis of the topological and energetic properties of the electron density distribution involving X–H···F–Y systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Churakov, A.V.; Tsirelson, V.G. Intermolecular hydrogen bond energies in crystals evaluated using electron density properties: DFT computations with periodic boundary conditions. J. Comput. Chem. 2012, 33, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Bartashevich, E.V.; Tsirelson, V.G. Interplay between non-covalent interactions in complexes and crystals with halogen bonds. Russ. Chem. Rev. 2014, 83, 1181–1203. [Google Scholar] [CrossRef]
- Puttreddy, R.; von Essen, C.; Peuronen, A.; Lahtinen, M.; Rissanen, K. Halogen bonds in 2,5-dihalopyridine-copper(ii) chloride complexes. CrystEngComm 2018, 20, 1954–1959. [Google Scholar] [CrossRef]


| Contact | ρ(r) | ∇2ρ(r) | λ2 | Hb | V(r) | G(r) | Eint a | Eint b | Eint c | Eint d |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ||||||||||
| Cl···Cl, 3.505 Å | 0.007 | 0.023 | −0.009 | 0.001 | −0.003 | 0.005 | 0.9 | 1.3 | 0.9 | 1.5 |
| Cl···Cl, 3.543 Å | 0.006 | 0.021 | −0.008 | 0.001 | −0.003 | 0.004 | 0.9 | 1.1 | 0.9 | 1.2 |
| 2 | ||||||||||
| Br···Cl, 3.401 Å | 0.009 | 0.030 | −0.012 | 0.001 | −0.005 | 0.006 | 1.6 | 1.6 | 1.8 | 2.1 |
| Br···Cl, 3.418 Å | 0.009 | 0.029 | −0.012 | 0.001 | −0.005 | 0.006 | 1.6 | 1.6 | 1.8 | 2.1 |
| 3 | ||||||||||
| I···Cl, 3.393 Å | 0.012 | 0.041 | −0.016 | 0.001 | −0.008 | 0.009 | 2.5 | 2.4 | 3.4 | 3.8 |
| I···Cl, 3.400 Å | 0.012 | 0.040 | −0.015 | 0.001 | −0.008 | 0.009 | 2.5 | 2.4 | 3.4 | 3.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adonin, S.A.; Bondarenko, M.A.; Novikov, A.S.; Sokolov, M.N. Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines. Crystals 2020, 10, 289. https://doi.org/10.3390/cryst10040289
Adonin SA, Bondarenko MA, Novikov AS, Sokolov MN. Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines. Crystals. 2020; 10(4):289. https://doi.org/10.3390/cryst10040289
Chicago/Turabian StyleAdonin, Sergey A., Mikhail A. Bondarenko, Alexander S. Novikov, and Maxim N. Sokolov. 2020. "Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines" Crystals 10, no. 4: 289. https://doi.org/10.3390/cryst10040289
APA StyleAdonin, S. A., Bondarenko, M. A., Novikov, A. S., & Sokolov, M. N. (2020). Halogen Bonding in Isostructural Co(II) Complexes with 2-Halopyridines. Crystals, 10(4), 289. https://doi.org/10.3390/cryst10040289