Magnetic and Luminescent Properties of Isostructural 2D Coordination Polymers Based on 2-Pyrimidinecarboxylate and Lanthanide Ions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.1.1. Synthesis of [Dy4(pymca)4(AcO)8]n (1)
2.1.2. Synthesis of [Nd4(pymca)4(AcO)8]n (2)
2.2. Characterization Methods
2.2.1. Physico-Chemical Characterization
2.2.2. Single-Crystal X-Ray Diffraction
2.2.3. Magnetic Measurements
2.2.4. Luminescent Measurements
2.2.5. Computational Details
3. Results and Discussion
3.1. Crystal Structure of [Ln4(pymca)4(AcO)8]n (Ln = Dy (1), Nd (2))
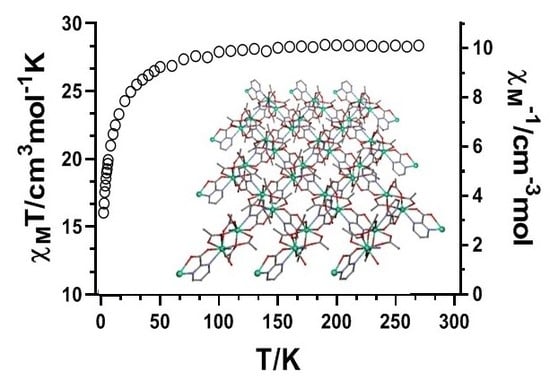
3.2. Magnetic Properties of Compound 1
3.2.1. Static Magnetic Measurements
3.2.2. Dynamic Magnetic Properties
3.3. Luminescence Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robin, A.Y.; Fromm, K.M. Coordination polymer networks with O- and N-donors: What they are, why and how they are made. Coord. Chem. Rev. 2006, 250, 2127–2157. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Zhang, J.; Chen, Y.-M.; Yang, G.-Y. Porous Lanthanide–Organic Open Frameworks with Helical Tubes Constructed from Interweaving Triple-Helical and Double-Helical Chains. Angew. Chem. Int. Ed. 2005, 44, 5814–5817. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Huang, X.; Li, J.; Wu, Y.; Zheng, N. Novel Single- and Double-Layer and Three-Dimensional Structures of Rare-Earth Metal Coordination Polymers: The Effect of Lanthanide Contraction and Acidity Control in Crystal Structure Formation. Angew. Chem. Int. Ed. 2000, 39, 527–530. [Google Scholar] [CrossRef]
- Pan, L.; Adams, K.M.; Hernandez, H.E.; Wang, X.; Zheng, C.; Hattori, Y.; Kaneko, K. Porous Lanthanide-Organic Frameworks: Synthesis, Characterization, and Unprecedented Gas Adsorption Properties. J. Am. Chem. Soc. 2003, 125, 3062–3067. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ma, C.; Song, Y.; Xu, F.; Chen, S.; Wang, L. Determination of tetracycline in milk by using nucleotide/lanthanide coordination polymer-based ternary complex. J. Biosens. Bioelectron. 2013, 50, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Chen, X.-Y.; Cheng, P.; Liao, D.-Z.; Yan, S.-P.; Jiang, Z.-H. Coordination Polymers Containing 1D Channels as Selective Luminescent Probes. J. Am. Chem. Soc. 2004, 126, 15394–15395. [Google Scholar] [CrossRef]
- Andruh, M.; Costes, J.-P.; Diaz, C.; Gao, S. 3d-4f Combined Chemistry: Synthetic Strategies and Magnetic Properties. Inorg. Chem. 2009, 48, 3342–3359. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Xia, J. Synthesis, Structures, and Properties of Lanthanide Complexes with Pyrimidine-2-carboxylic Acid. Z. Anorg. Allg. Chem. 2017, 643, 1752–1758. [Google Scholar] [CrossRef]
- Li, B.; Wen, H.-M.; Cui, Y.; Qian, G.; Chen, B. Multifunctional lanthanide coordination polymers. Prog. Polym. Sci. 2015, 48, 40–84. [Google Scholar] [CrossRef]
- Colacio, E.; Rodríguez-Diéguez, A. Possibilities with 2-pyrimidinecarbonitrile to design MOFs. Polyhedron 2014, 80, 173–179. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Yue, Q.; Jia, Q.-X.; Cheng, A.-L.; Gao, E.-Q. Syntheses, structures and luminescence properties of cadmium (II) coordination polymers with in situ formed oxalate and bis(chelating) bridging ligands. Cryst. Eng. Comm. 2008, 10, 1443–1449. [Google Scholar] [CrossRef]
- Zhang, X.-M.; Zheng, Y.-Z.; LI, C.-R.; Zhang, W.-X.; Chen, X.-M. Unprecedented (3,9)-Connected (42.6)3(46.621.89) Net Constructed by Trinuclear Mixed-Valence Cobalt Clusters. Cryst. Growth Des. 2007, 7, 980–983. [Google Scholar] [CrossRef]
- Long, D.-L.; Blake, A.J.; Champness, N.R.; Wilson, C.; Schröder, M. Unprecedented Seven- and Eight-Connected Lanthanide Coordination Networks. Angew. Chem. Int. Ed. 2001, 40, 2443–2447. [Google Scholar] [CrossRef]
- Sun, R.; Gao, S.-M.; Wang, S.-Q.; Song, W.-C.; Zhao, J.-P.; Liu, F.-C. Co-ligand tuned pyrimidine-2-carboxylate Mn (II) complexes from a 2D 63 layer to an interpenetrated srs-net. Dalton Trans. 2017, 46, 8593–8597. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, L.; Fan, Y.; Wang, L.; Xu, J. Construction of a series of lanthanide metal–organic frameworks: Synthesis, structure, luminescence and white light emission. Cryst. Eng. Comm. 2015, 17, 9363–9369. [Google Scholar] [CrossRef]
- Jia, L.; Sun, H.-L.; Wang, Z. Crystal structures and luminescent properties of new lanthanide(III) complexes derived from 2-phenyl-4-pyrimidinecarboxylate. RSC Adv. 2015, 5, 96855–96861. [Google Scholar] [CrossRef]
- Wang, Z.-N.; Xu, X.-T.; Lv, X.; Bai, F.-Y.; Liu, S.-Q.; Xing, Y.-H. Synthesis, crystal structure, fluorescence and antimicrobial activity of a series of rare-earth complexes based on indolebutyric acid. RSC Adv. 2015, 5, 104263–104274. [Google Scholar] [CrossRef]
- Rodríguez-Diéguez, A.; Cano, J.; Kivekäs, R.; Debdoubi, A. and Colacio, Self-Assembled Cationic Heterochiral Honeycomb-Layered Metal Complexes with the in Situ Generated Pyrimidine-2-carboxylato Bisdidentate Ligand. Hydrothermal Synthesis, Crystal Structures, Magnetic Properties, and Theoretical Study of [M2 (µ-pymca)3]OH∙H2O (M = FeII, CoII). Inorg. Chem. 2007, 46, 2503–2510. [Google Scholar] [CrossRef]
- Suárez-Varela, J.; Mota, A.J.; Aouryaghal, H.; Cano, J.; Rodríguez-Diéguez, A.; Luneau, D.; Colacio, E. Anion Influence on the Structure and Magnetic Properties of a Series of Multidimensional Pyrimidine-2-carboxylato-Bridged Copper(II) Complexes. Inorg. Chem. 2008, 47, 8143–8158. [Google Scholar] [CrossRef]
- Colacio, E.; Aouryaghal, H.; Mota, A.J.; Cano, J.; Sillanpääe, R.; Rodríguez-Diéguez, A. Anion encapsulation promoted by anion/p interactions in rationally designed hexanuclear antiferromagnetic wheels: Synthesis, structure and magnetic properties. Cryst. Eng. Comm. 2009, 11, 2054–2064. [Google Scholar] [CrossRef]
- Oyarzabal, I.; Ruiz, J.; Ruiz, E.; Aravena, D.; Seco, J.M.; Colacio, E. Increasing the effective energy barrier promoted by the change of a counteranion in a Zn-Dy-Zn SMM: Slow relaxation via the second excited state. Chem. Commun. 2015, 51, 12353–12356. [Google Scholar] [CrossRef] [Green Version]
- Bruker. Bruker AXS Inc. V2019.1; Bruker: Madison, WI, USA, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.; Bourhis, L.J.; Gildea, R.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, E.; Cano, J.; Álvarez, S.; Alemany, P. Broken symmetry approach to calculation of exchange coupling constants for homobinuclear and heterobinuclear transition metal complexes. J. Comput. Chem. 1999, 20, 1391–1400. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision A.01; Gaussian, Inc.: Wallingford, UK, 2016. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView; Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Chilton, N.F.; Collison, D.; McInnes, E.J.; Winpenny, R.E.; Soncini, A. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes. Nat. Commun. 2013, 4, 2551–2558. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. cclib: A library for package-independent computational chemistry algorithms. J. Comput. Chem. 2008, 29, 839–845. [Google Scholar] [CrossRef]
- Ruiz, C.; García-Valdivia, A.A.; Fernández, B.; Cepeda, J.; Oyarzabal, I.; Abas, E.; Laguna, M.; García, J.A.; Fernández, I.; San Sebastian, E.; et al. Multifunctional coordination compounds based on lanthanide ions and 5-bromonicotinic acid: Magnetic, luminescence and anti-cancer properties. CrystEngComm 2019, 21, 3881–3890. [Google Scholar] [CrossRef]
- Noodleman, W.-G.H. Structural model studies for the high-valent intermediate Q ofmethane monooxygenase from broken-symmetry density functional calculations. Inorg. Chim. Acta 2008, 361, 973–986. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, J.; Mota, A.J.; Rodríguez-Diéguez, A.; Titos, S.; Herrera, J.M.; Ruiz, E.; Cremades, E.; Costes, J.P.; Colacio, E. Field and dilution effects on the slow relaxation of a luminescent DyO9 low-symmetry single-ion magnet. Chem. Commun. 2012, 48, 7916–7918. [Google Scholar] [CrossRef] [PubMed]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Zheng, Y.-Z.; Lin, W.-Q.; Ungur, L.; Wernsdorfer, W.; Chibotaru, L.F.; Tong, M.-L. Switching the anisotropy barrier of a single-ion magnet by symmetry change from quasi-D5h to quasi-Oh. Chem. Sci. 2013, 4, 3310–3316. [Google Scholar] [CrossRef]
- García-Valdivia, A.; Zabala-Lekuona, A.; Goñi-Cárdenas, A.; Fernández, B.; García, J.A.; Quílez del Moral, J.F.; Cepeda, J.; Rodríguez-Diéguez, A. Dilution Effect on the Slow Relaxation of a Luminescent Dysprosium Metal-Organic Framework based on 2, 5-dihydroxyterephthalic acid. Inorg. Chim. Acta 2020, 509, 119687. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- Heine, J.; Müller-Buschbaum, K. Engineering metal-based luminescence in coordination polymers and metal–organic frameworks. Chem. Soc. Rev. 2013, 42, 9232–9242. [Google Scholar] [CrossRef]








| Compound | 1 | 2 |
|---|---|---|
| Formula | C18H18N4O12Dy2 | C18H18N4O12Nd2 |
| Mr (g moL−1) | 807.36 | 770.84 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21 | P21 |
| Temperature (K) | 298(2) | 100 |
| a (Å) | 15.0967(7) | 15.1628(7) |
| b (Å) | 9.3786(4) | 9.5069(4) |
| c (Å) | 16.4416(8) | 16.6857(8) |
| α (°) | 90 | 90 |
| β (°) | 103.8920(10) | 104.791(2) |
| λ (°) | 90 | 90 |
| V (Å3) | 2259.81(18) | 2325.56(18) |
| Z | 4 | 4 |
| ρ (g cm−3) | 2.373 | 2.202 |
| µ (mm−1) | 6.635 | 4.487 |
| Unique reflections | 10,291 | 7722 |
| Rint | 0.057 | 0.088 |
| GoF a | 1.119 | 1.023 |
| R1 b/wR2 c [I > 2σ(I)] | 0.0590/0.1481 | 0.0750/0.1740 |
| R1 b/wR2 c [all data] | 0.0823/0.1624 | 0.1279/02049 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, A.; Zabala-Lekuona, A.; Goñi-Cárdenas, A.; Cepeda, J.; Seco, J.M.; Salinas-Castillo, A.; Choquesillo-Lazarte, D.; Rodríguez-Diéguez, A. Magnetic and Luminescent Properties of Isostructural 2D Coordination Polymers Based on 2-Pyrimidinecarboxylate and Lanthanide Ions. Crystals 2020, 10, 571. https://doi.org/10.3390/cryst10070571
García-García A, Zabala-Lekuona A, Goñi-Cárdenas A, Cepeda J, Seco JM, Salinas-Castillo A, Choquesillo-Lazarte D, Rodríguez-Diéguez A. Magnetic and Luminescent Properties of Isostructural 2D Coordination Polymers Based on 2-Pyrimidinecarboxylate and Lanthanide Ions. Crystals. 2020; 10(7):571. https://doi.org/10.3390/cryst10070571
Chicago/Turabian StyleGarcía-García, Amalia, Andoni Zabala-Lekuona, Ainhoa Goñi-Cárdenas, Javier Cepeda, José M. Seco, Alfonso Salinas-Castillo, Duane Choquesillo-Lazarte, and Antonio Rodríguez-Diéguez. 2020. "Magnetic and Luminescent Properties of Isostructural 2D Coordination Polymers Based on 2-Pyrimidinecarboxylate and Lanthanide Ions" Crystals 10, no. 7: 571. https://doi.org/10.3390/cryst10070571
APA StyleGarcía-García, A., Zabala-Lekuona, A., Goñi-Cárdenas, A., Cepeda, J., Seco, J. M., Salinas-Castillo, A., Choquesillo-Lazarte, D., & Rodríguez-Diéguez, A. (2020). Magnetic and Luminescent Properties of Isostructural 2D Coordination Polymers Based on 2-Pyrimidinecarboxylate and Lanthanide Ions. Crystals, 10(7), 571. https://doi.org/10.3390/cryst10070571