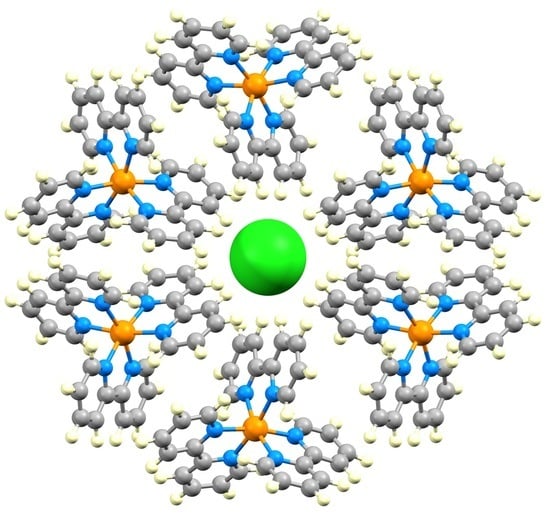
Halide Ion Embraces in Tris(2,2′-bipyridine)metal Complexes
Abstract
:1. Introduction
2. The Choice of Metal Complex Scaffold
2.1. The Oligopyridines
2.2. Supramolecular Interactions in Oligopyridine Complexes
2.3. Outer-Sphere Complexes and Ion-Pairing
2.4. Methodology
3. Ubiquitous Interactions in [M(bpy)3]n+ Complexes
3.1. The Structural Motifs
3.2. A Closer Look at Structure Type 1
3.3. An Aside on Chirality
3.4. From 2,2′-bipyridine to 1,10-phenanthroline
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- ICSD FIZ Karlsruhe–Leibnitz Institute for Information Infrastructure. Available online: https://www.european-mrs.com/fiz-karlsruhe-%E2%80%93-leibniz-institute-information-infrastructure (accessed on 20 March 2020).
- NIST Inorganic Crystal Structure Database, NiISTStandard Reference Database Number 3, National Institute of Standards and Technology, Gaithersburg Md, 20899. Available online: https://www.nist.gov/programs-projects/crystallographic-databases (accessed on 20 March 2020).
- PDBE Protein Data Bank in Europe. Available online: https://www.ebi.ac.uk/pdbe/ (accessed on 20 March 2020).
- RCSB Protein Data Bank. Available online: https://www.rcsb.org (accessed on 20 March 2020).
- PDBJ Protein Data Bank Japan. Available online: https://pdbj.org (accessed on 20 March 2020).
- CCDC the Cambridge Structural Database (CSD). Available online: https://www.ccdc.cam.ac.uk (accessed on 20 March 2020).
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, 72B, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualizing crystal structures. Acta Crystallogr. 2002, 58B, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.E.; Bolink, H.J.; Constable, E.C.; Ertl, C.D.; Housecroft, C.E.; Pertegàs, A.; Zampese, J.A.; Kanitz, A.; Kessler, F.; Meier, S.B. Chloride ion impact on materials for light-emitting electrochemical cells. Dalton Trans. 2014, 43, 1961–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constable, E.C.; Housecroft, C.E. More Hydra than Janus—Non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C.; Seddon, K.R. A deuterium exchange reaction of the tris-(2,2′-bipyridine)ruthenium(II) cation: Evidence for the acidity of the 3,3′-protons. J. Chem. Soc. Chem. Commun. 1982, 34–36. [Google Scholar] [CrossRef]
- Constable, E.C.; Lewis, J. Nmr studies on ruthenium(ii) α,α′-diimine complexes; further evidence for unique reactivity at H3,3′ of coordinated 2,2′-bipyridines. Inorg. Chim. Acta 1983, 70, 251–253. [Google Scholar] [CrossRef]
- McClanahan, S.; Hayes, T.; Kincaid, J. Resonance Raman spectra of the ground and excited states of specifically deuterated tris(2,2′-bipyridine)ruthenium(II). J. Am. Chem. Soc. 1983, 105, 4486–4487. [Google Scholar] [CrossRef]
- McClanahan, S.; Kincaid, J. Vibrational spectra of specifically deuteriated 2,2′-bipyridine complexes of Fe(II), Ru(II) and Os(II). J. Raman Spectrosc. 1984, 15, 173–178. [Google Scholar] [CrossRef]
- McClanahan, S.F.; Kincaid, J.R. 3MLCT lifetimes of tris(2,2′-bipyridine)ruthenium(II). Position-dependent deuterium effects. J. Am. Chem. Soc. 1986, 108, 3840–3841. [Google Scholar] [CrossRef]
- Wernberg, O. A kinetic investigation of the base-catalysed deuterium-exchange reaction of tris(2,2′-bipyridine)osmium(II) ion in dimethyl sulphoxide solution. J. Chem. Soc. Dalton Trans. 1986, 1993–1994. [Google Scholar] [CrossRef]
- Constable, E.C. Ligand reactivity in 2,2′-bipyridine complexes; charge effects upon reactions with base. Polyhedron 1989, 8, 83–86. [Google Scholar] [CrossRef]
- Butschke, B.; Schwarz, H. “Rollover” cyclometalation—Early history, recent developments, mechanistic insights and application aspects. Chem. Sci. 2012, 3, 308–326. [Google Scholar] [CrossRef] [Green Version]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; Oxford University Press: Oxford, UK, 1999. [Google Scholar]
- Broder, C.K.; Davidson, M.G.; Forsyth, V.T.; Howard, J.A.K.; Lamb, S.; Mason, S.A. On the reliability of C−H···O interactions in crystal engineering: synthesis and structure of two hydrogen bonded phosphonium bis(aryloxide) salts. Cryst. Growth Des. 2002, 2, 163–169. [Google Scholar] [CrossRef]
- Desiraju, G.R. The Crystal as a Supramolecular Entity; Wiley: Chichester, UK, 1996. [Google Scholar]
- Brandt, W.W.; Dwyer, F.P.; Gyarfas, E.D. Chelate complexes of 1,10-phenanthroline and related compounds. Chem. Rev. 1954, 54, 959–1017. [Google Scholar] [CrossRef]
- Constable, E.C. The coordination chemistry of 2,2′:6′,2″-terpyridine and higher oligopyridines. Adv. Inorg. Chem. 1986, 30, 69–121. [Google Scholar] [CrossRef]
- Constable, E.C. Homoleptic complexes of 2,2′-bipyridine. Adv. Inorg. Chem. 1989, 34, 1–63. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The early years of 2,2′-bipyridine—A ligand in its own lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C. 2,2′:6′,2″-terpyridines: From chemical obscurity to common supramolecular motifs. Chem. Soc. Rev. 2007, 36, 246–253. [Google Scholar] [CrossRef]
- Constable, E.C. Higher oligopyridines as a structural motif in metallosupramolecular chemistry. Prog. Inrog. Chem. 1994, 42, 67–138. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. ‘Simple’ oligopyridine complexes—Sources of unexpected structural diversity. Aust. J. Chem. 2020, 73, 390. [Google Scholar] [CrossRef]
- Fallahpour, R.-A. The higher oligopyridines and their metal complexes. Curr. Org. Synth. 2006, 3, 19–39. [Google Scholar] [CrossRef]
- Kaes, C.; Katz, A.; Hosseini, M.W. Bipyridine: The most widely used ligand. A review of molecules comprising at least two 2,2′-bipyridine units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef] [PubMed]
- Lindoy, L.F.; Livingstone, S.E. Complexes of iron(II), cobalt(II) and nickel(II) with α-diimines and related bidentate ligands. Coord. Chem. Rev. 1967, 2, 173–193. [Google Scholar] [CrossRef]
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Schubert, U.S.; Winter, A.; Newkome, G.R. Terpyridine-Based Materials; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Summers, L.A. The bipyridines. Adv. Heterocycl. Chem. 1984, 35, 281–374. [Google Scholar] [CrossRef]
- McWhinnie, W.R.; Miller, J.D. The chemistry of complexes containing 2,2′-bipyridyl, 1,10-phenanthroline, or 2,2′,6′,2”-terpyridyl as ligands. Adv. Inorg. Chem. Radiochem. 1970, 12, 135–215. [Google Scholar] [CrossRef]
- Constable, E.C. A journey from solution self-assembly to designed interfacial assembly. Adv. Inorg. Chem. 2018, 71, 79–134. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C. Metallosupramolecular chemistry. Chem. Ind. 1994, 56–59. [Google Scholar]
- Glasson, C.R.K.; Lindoy, L.F.; Meehan, G.V. Recent developments in the d-block metallo-supramolecular chemistry of polypyridyls. Coord. Chem. Rev. 2008, 252, 940–963. [Google Scholar] [CrossRef]
- Steel, P.J. Metallosupramolecular chemistry—What is it? ChemInform 2004, 35. [Google Scholar] [CrossRef] [Green Version]
- Dance, I.; Scudder, M. Supramolecular motifs: Sextuple aryl embraces in crystalline [M(2,2′-bipy)3] and related complexes. J. Chem. Soc. Dalton Trans. 1998, 1341–1350. [Google Scholar] [CrossRef]
- McMurtrie, J.; Dance, I. Alternative two-dimensional embrace nets formed by metal complexes of 4′-phenylterpyridine crystallised with hydrophilic anions. CrystEngComm 2010, 12, 3207–3217. [Google Scholar] [CrossRef]
- McMurtrie, J.; Dance, I. Crystal packing in metal complexes of 4′-phenylterpyridine and related ligands: Occurrence of the 2D and 1D terpy embrace arrays. CrystEngComm 2009, 11, 1141–1149. [Google Scholar] [CrossRef]
- McMurtrie, J.; Dance, I. Alternative metal grid structures formed by [M(terpy)2]2+ and [M(terpyOH)2]2+ complexes with small and large tetrahedral dianions, and by [Ru(terpy)2]0. CrystEngComm 2010, 12, 2700–2710. [Google Scholar] [CrossRef]
- Dance, I. Intermolecular embraces and intermolecular energies. Mol. Cryst. Liq. Cryst. 2005, 440, 265–293. [Google Scholar] [CrossRef]
- Maharaj, F.; Russell, V.; Chow, H.; Page, M.; Scudder, M.; Craig, D.; Dance, I. Polymorphs and pseudo-polymorphs: Nine crystals containing [Fe(phen)3]2+ associated with [HgI4]2–. CrystEngComm 2003, 5, 285–293. [Google Scholar] [CrossRef]
- McMurtrie, J.; Dance, I. Engineering grids of metal complexes: Development of the 2D M(terpy)2 embrace motif in crystals. CrystEngComm 2005, 7, 216–229. [Google Scholar] [CrossRef]
- McMurtrie, J.; Dance, I. Engineering the metal-terpy grid with complexes containing 4′-hydroxy terpyridine. CrystEngComm 2005, 7, 230–236. [Google Scholar] [CrossRef]
- Dance, I. Inorganic intermolecular motifs, and their energies. CrystEngComm 2003, 5, 208–221. [Google Scholar] [CrossRef]
- Dance, I. Distance criteria for crystal packing analysis of supramolecular motifs. New J. Chem. 2003, 27, 22–27. [Google Scholar] [CrossRef]
- Maharaj, F.; Russell, V.; Scudder, M.; Craig, D.; Dance, I. A highly symmetric lattice of tightly packed supramolecular interactions of [Fe(phen)3]2+ with [HgI3]−, [ClHgI3]2−, and Cl−. CrystEngComm 2002, 4, 149–154. [Google Scholar] [CrossRef]
- Horn, C.; Berben, L.; Chow, H.; Scudder, M.; Dance, I. Supramolecular motifs in four pseudo-polymorphic crystals of [Fe(phen)3](I3)2·(solvent): Solvent = acetone, CH2Cl2, CH3CN, toluene or H2O. CrystEngComm 2002, 4, 7–12. [Google Scholar] [CrossRef]
- Horn, C.; Scudder, M.; Dance, I. Crystal structures, crystal packing and supramolecular motifs in [Fe(phen)3]I14 and [M(phen)3]I18 (M⊕=⊕Fe, Ni): Complementary orthogonality of [M(phen)3]2+ cations and polyiodide anions. CrystEngComm 2001, 3, 9–14. [Google Scholar] [CrossRef]
- Horn, C.; Scudder, M.; Dance, I. Contrasting crystal supramolecularity for [Fe(phen)3]I8 and [Mn(phen)3]I8: Complementary orthogonality and complementary helicity. CrystEngComm 2001, 3, 1–8. [Google Scholar] [CrossRef]
- Horn, C.; Scudder, M.; Dance, I. The crystal packing of [M(phen)3]I7 (M = Mn, Fe). CrystEngComm 2000, 2, 196–200. [Google Scholar] [CrossRef]
- Russell, V.; Scudder, M.; Dance, I. The crystal supramolecularity of metal phenanthroline complexes. Dalton Trans. 2001, 789–799. [Google Scholar] [CrossRef]
- Horn, C.; Scudder, M.; Dance, I. Crystal supramolecular motifs in trimorphs of [Fe(phen)3]I12. CrystEngComm 2000, 2, 53–66. [Google Scholar] [CrossRef]
- Horn, C.; Ali, B.; Dance, I.; Scudder, M.; Craig, D. Crystal supramolecularity: Extended aryl embraces in dimorphs of [Cu(1,10-phen)2I]I3. CrystEngComm 2000, 2, 6–15. [Google Scholar] [CrossRef]
- Nassimbeni, L.R.; Bond, D.R.; Moore, M.; Papanicolaou, S. Structure-energy relationships of Werner clathrates. Acta Crystallogr. 1984, 40A, C111. [Google Scholar] [CrossRef] [Green Version]
- Noa, F.M.A.; Bourne, S.A.; Nassimbeni, L.R. Hydrogen bonding and secondary interactions in halogenated complexes. Acta Crystallogr. 2017, 73A, C736. [Google Scholar] [CrossRef] [Green Version]
- Noa, F.M.A.; Bourne, S.A.; Su, H.; Weber, E.; Nassimbeni, L.R. Hydrogen bonding versus halogen bonding in host guest compounds. Cryst. Growth Des. 2016, 16, 4765–4771. [Google Scholar] [CrossRef]
- Noa, F.M.A.; Bourne, S.A.; Su, H.A.; Nassimbeni, L.R. Secondary interactions in halogenated Werner clathrates. Cryst. Growth Des. 2017, 17, 1876–1883. [Google Scholar] [CrossRef]
- Sykes, N.M.; Su, H.; Bourne, S.A.; Nassimbeni, L.R. Enclathration by Werner hosts: Selectivity and polymorphism. Cryst. Growth Des. 2020, 20, 274–280. [Google Scholar] [CrossRef]
- Wicht, M.; Bathori, N.; Nassimbeni, L. Mixed-ligand Ni–Werner complexes: Enhanced selectivity and hydrogen bonding frameworks. Acta Crystallogr. 2018, 74A, E130. [Google Scholar] [CrossRef]
- Wicht, M.M.; Nassimbeni, L.R.; Bathori, N.B. Werner clathrates with enhanced hydrogen bonding functionality. Polyhedron 2019, 163, 7–19. [Google Scholar] [CrossRef]
- Beck, M.T. Chemistry of the outer-sphere complexes. Coord. Chem. Rev. 1968, 3, 91–115. [Google Scholar] [CrossRef]
- Eaton, D.R. Outer sphere complexes as intermediates in coordination chemistry. Rev. Chem. Intermed. 1988, 9, 201–232. [Google Scholar] [CrossRef]
- Bizunok, M.B.; Pyartman, A.K.; Mironov, V.E. Interaction of tris(2,2′-dipyridylchromium(III)) with chloride-, bromide-, nitrate-, iodide-, and sulfate ions in aqueous solutions. Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. 1983, 26, 907–910. [Google Scholar]
- Chattopadhyay, P.K.; Coetzee, J.F. Influence of anionic inner-sphere substituents on the kinetics of ternary complex formation of nickel(II) in acetonitrile as solvent. Opposing effects of solvent labilization and outer-sphere destabilization. Inorg. Chem. 1976, 15, 400–405. [Google Scholar] [CrossRef]
- Coetzee, J.F.; Gilles, D.M. Binary and ternary complex formation of nickel(II) in methanol. Further evidence for outer-sphere stabilization and other factors contributing to ligand substitution kinetics in nonaqueous solvents. Inorg. Chem. 1976, 15, 405–408. [Google Scholar] [CrossRef]
- Fox, D.; Wells, C.F. Kinetics of the oxidation of chloride ions by tris(2,2′-bipyridine)nickel(III) ions in aqueous perchlorate media. J. Chem. Soc. Dalton Trans. 1989, 151–154. [Google Scholar] [CrossRef]
- Kameta, N.; Imura, H.; Ohashi, K. Stability constants of inner- and outer-sphere complexes of hydrated tris(1-(2-thienyl)-4,4,4-trifluoro-1,3-butanedionato) lanthanide(III) with tris(2,4-pentanedionato) cobalt(III). Polyhedron 2002, 21, 805–810. [Google Scholar] [CrossRef]
- Ilcheva, L.; Beck, M.T. Effect of ionic strength on the stability of outer sphere complexes. Magy. Kem. Foly. 1974, 80, 132–135. [Google Scholar]
- Ilcheva, L.; Beck, M.T. The effect of ionic strength on the stability outer sphere complexes. Acta Chim. Acad. Sci. Hung. 1978, 97, 45–49. [Google Scholar]
- Johansson, L. Outer-sphere complexes of the tris(1,10-phenanthroline)iron(II) and tris(2,2′-bipyridine)iron(II) ions with several anions. Chem. Scr. 1976, 10, 72–75. [Google Scholar]
- Langford, C.H.; Vuik, C.P.J.; Kane-Maguire, N.A.P. Ligand field redox photochemistry of phenanthroline and bipyridine cobalt(III) complexes. Oxalate as an inner and outer sphere reducing agent. Inorg. Nucl. Chem. Lett. 1975, 11, 377–380. [Google Scholar] [CrossRef]
- Pyartman, A.K.; Gromova, G.I.; Mironov, V.E. Reactions of mixed complexing and substitution of ligands in the second sphere of tris(2,2′-dipyridyl)iron(II). Halide compounds. Koord. Khim. 1980, 6, 443–445. [Google Scholar]
- Pyartman, A.K.; Gromova, G.I.; Mironov, V.E. Reactions of mixed complexing and substitution of ligands in the second sphere of tris(2,2′-dipyridyl)iron(II). Perchlorate-halide compounds. Koord. Khim. 1980, 6, 439–442. [Google Scholar]
- Pov-Ray. Available online: http://www.povray.org (accessed on 20 March 2020).
- Tada, T. Optical resolution of tris(2,2′-bipyridine)metal(II) by d-tartrate. J. Sci. Hiroshima Univ. Ser. A Phys. Chem. 1982, 46, 73–93. [Google Scholar]
- Otsuka, T.; Takahashi, N.; Fujigasaki, N.; Sekine, A.; Ohashi, Y.; Kaizu, Y. Crystal structure and energy transfer in double-complex salts composed of tris(2,2′-bipyridine)ruthenium(II) or tris(2,2′-bipyridine)osmium(II) and hexacyanochromate(III). Inorg. Chem. 1999, 38, 1340–1347. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Liu, X.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P.; Yao, X.-K.; Wang, G.-L. A novel complex of sandwich layers having disordered symbiotic distribution of azido group and water molecules. Inorg. Chem. Comm. 2001, 4, 416–418. [Google Scholar] [CrossRef]
- Zhang, C.-D.; Liu, S.-X.; Gao, B.; Sun, C.-Y.; Xie, L.-H.; Yu, M.; Peng, J. Hybrid materials based on metal–organic coordination complexes and cage-like polyoxovanadate clusters: Synthesis, characterization and magnetic properties. Polyhedron 2007, 26, 1514–1522. [Google Scholar] [CrossRef]
- Dong, B.; Peng, J.; Chen, Y.; Kong, Y.; Tian, A.; Liu, H.; Sha, J. Ph-controlled assembly of two polyoxovanadates based on [V16O38(Cl)]8− and [V15O36(Cl)]6− building blocks. J. Mol. Struct. 2006, 788, 200–205. [Google Scholar] [CrossRef]
- Dong, B.; Peng, J.; Tian, A.; Sha, J.; Li, L.; Liu, H. Two new inorganic–organic hybrid single pendant hexadecavanadate derivatives with bifunctional electrocatalytic activities. Electrochim. Acta 2007, 52, 3804–3812. [Google Scholar] [CrossRef]
- Shi, S.-Y.; Chen, Y.; Liu, B.; Lu, Y.-K.; Xu, J.-N.; Cui, X.-B.; Xu, J.-Q. Two supramolecular compounds based on cage-like polyoxovanadates: Syntheses, crystal structures, and characterizations. J. Coord. Chem. 2009, 62, 2937–2948. [Google Scholar] [CrossRef]
- Li, Y.-G.; Lu, Y.; Luan, G.-Y.; Wang, E.-B.; Duan, Y.-B.; Hu, C.-W.; Hu, N.-H.; Jia, H.-Q. Hydrothermal syntheses and crystal structures of new cage-like mixed-valent polyoxovanadates. Polyhedron 2002, 21, 2601–2608. [Google Scholar] [CrossRef]
- Low, K.S.; Cole, J.M.; Zhou, X.; Yufa, N. Rationalizing the molecular origins of Ru- and Fe-based dyes for dye-sensitized solar cells. Acta Crystallogr. 2012, 68B, 137–149. [Google Scholar] [CrossRef]
- Lei, X.W.; Yue, C.Y.; Zhao, J.Q.; Han, Y.F.; Yang, J.T.; Meng, R.R.; Gao, C.S.; Ding, H.; Wang, C.Y.; Chen, W.D.; et al. Two types of 2d layered iodoargentates based on trimeric [Ag3I7] secondary building units and hexameric [Ag6I12] ternary building units: Syntheses, crystal structures, and efficient visible light responding photocatalytic properties. Inorg. Chem. 2015, 54, 10593–10603. [Google Scholar] [CrossRef]
- Ruiz-Pérez, C.; Lorenzo Luis, P.A.; Lloret, F.; Julve, M. Dimensionally controlled hydrogen-bonded nanostructures: Synthesis, structure, thermal and magnetic behaviour of the tris-(chelated)nickel(II) complex [Ni(bipy)3Cl2·5.5H2O (bipy = 2,2′-bipyridyl). Inorg. Chim. Acta 2002, 336, 131–136. [Google Scholar] [CrossRef]
- Heilmann, J.; Lerner, H.-W.; Bolte, M. Tris(2,2′-bipyridyl)iron(II) dibromide 4.5-hydrate. Acta Crystallogr 2006, 62E, m1477–m1478. [Google Scholar] [CrossRef]
- Fischer, E.; Hummel, H.-U. Untersuchungen im quasi-binären System LiI/2,2′-Bipyridin. Z. Anorg. Allgem. Chem. 1997, 623, 483–486. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, J.-F.; Bei, F.-L.; Zhang, C.; Yang, J.-Y.; Song, Y.-L. Two new configurations of 1-d heterothiometallic clusters: Isomerism of polymeric anions induced by both complementary cations and anions. Inorg. Chem. Comm. 2007, 10, 1214–1217. [Google Scholar] [CrossRef]
- Tada, T.; Kushi, Y.; Yoneda, H. Three types of diastereomeric salts containing tris-(2,2′-bipyridine)metal(II) complex and d-tartrate ions. Inorg. Chim. Acta 1982, 64, L243–L245. [Google Scholar] [CrossRef]
- Wada, A.; Katayama, C.; Tanaka, J. The crystal structure and absolute configuration of (+)589-tris(2,2′-bipyridyl)nickel(II) chloride (+)589-tartrate hydrate, [Ni(C10H8N2)3]2Cl2.C4H4O6.nH2O. Acta Crystallogr. 1976, 32B, 3194–3199. [Google Scholar] [CrossRef]
- Szalda, D.J.; Creutz, C.; Mahajan, D.; Sutin, N. Electron-transfer barriers and metal-ligand bonding as a function of metal oxidation state. 2. Crystal and molecular structures of tris(2,2′-bipyridine)cobalt(II) dichloride-2-water-ethanol and tris(2,2′-bipyridine)cobalt(I) chloride-water. Inorg. Chem. 1983, 22, 2372–2379. [Google Scholar] [CrossRef]
- Seifullina, I.; Martsinko, E.; Chebanenko, E.; Afanasenko, E.; Dyakonenko, V.; Shishkina, S. Supramolecular organization and structure of Cu(II) and Ni(II), 2,2′-bipyridine cations with tartratogermanate anions. Polyhedron 2019, 169, 261–265. [Google Scholar] [CrossRef]
- Sun, J.; Xu, H. Supramolecular assembly of [Co2(2,2′-bpy)6]·(BTCA)·Cl·11H2O: 3D negatively charged cages. Inorg. Chem. Comm. 2011, 14, 254–257. [Google Scholar] [CrossRef]
- Wood, P.A.; Scott, R.; Brechin, E. CSD Communication, 2015. (CCDC 1418045).
- Doğan, D.; Çolak, A.T.; Şahin, O.; Tunç, T.; Çelik, Ö. The syntheses, crystal structures, spectroscopic and thermal characterization of new pyridine-2,5-dicarboxylate compounds. Polyhedron 2015, 93, 37–45. [Google Scholar] [CrossRef]
- Papadopoulos, C.D.; Hatzidimitriou, A.G.; Voutsas, G.P.; Lalia-Kantouri, M. Synthesis and characterization of new addition compounds of bis(substituted-salicylaldehydo) cobalt(II) with 2,2′-bipyridine (bipy). Crystal and molecular structures of [CoII(3-methoxy-salicylaldehyde)2(bipy)]·CH3OH (1) and [CoII(bipy)3]Br2·0.5(5-chloro-salicylaldehydeH)·1.5CH3OH (5). Polyhedron 2007, 26, 1077–1086. [Google Scholar] [CrossRef]
- Yue, C.Y.; Lei, X.W.; Han, Y.F.; Lu, X.X.; Tian, Y.W.; Xu, J.; Liu, X.F.; Xu, X. Transition-metal-complex cationic dyes photosensitive to two types of 2d layered silver bromides with visible-light-driven photocatalytic properties. Inorg. Chem. 2016, 55, 12193–12203. [Google Scholar] [CrossRef]
- Puttreddy, R.; Hutchison, J.A.; Gorodetski, Y.; Harrowfield, J.; Rissanen, K. Enantiomer separation of tris(2,2′-bipyridine)ruthenium(II): Interaction of a D3-symmetric cation with a C2-symmetric anion. Cryst. Growth Des. 2015, 15, 1559–1563. [Google Scholar] [CrossRef]
- Hubesch, B.; Mahieu, B.; Meunier-Piret, J. Médiation électronique et effet de température dans le cycle photocatalytique du tris(2,2′-bipyridine)rhodium(III). Bull. Soc. Chim. Belg. 2010, 94, 685–696. [Google Scholar] [CrossRef]
- Liu, W.; Xu, W.; Lin, J.L.; Xie, H.Z. Tris(2,2′-bipyridine-κN:N′)cobalt(III) trichloride tetra-hydrate. Acta Crystallogr. 2008, 64E, m1586. [Google Scholar] [CrossRef] [Green Version]
- Casado, F.J.M.; Riesco, M.R. CSD Communication, 2010. (CCDC 768832).
- Tamura, H.; Ikeda, N.; Iguro, T.; Ohno, T.; Matsubayashi, G.E. The pseudo-racemic complex bis[tris(2,2′-bipyridine)ruthenium(II)] hexacyanocobaltate(III) chloride octahydrate, [Ru(bpy)3]2[Co(CN)6]Cl.8H2O. Acta Crystallogr. 1996, 52C, 1394–1399. [Google Scholar] [CrossRef]
- Marsh, R.E.; Spek, A.L. Use of software to search for higher symmetry: Space group C2. Acta Crystallogr. 2001, 57B, 800–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otsuka, T.; Sekine, A.; Fujigasaki, N.; Ohashi, Y.; Kaizu, Y. Energy-transfer rate in crystals of double-complex salts composed of [Ru(N-N)3]2+ (N-N = 2,2′-bipyridine or 1,10-phenanthroline) and [Cr(CN)6]3–: Effect of relative orientation between donor and acceptor. Inorg. Chem. 2001, 40, 3406–3412. [Google Scholar] [CrossRef]
- Sakai, K.; Uchida, Y.; Kajiwara, T.; Ito, T. Bis[tris(2,2′-bipyridine-kappa2N,N′)ruthenium(II)] hexacyanoferrate(III) chloride octahydrate. Acta Crystallogr. 2004, 60C, m65–m68. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.J.; Jones, C.; Kloth, M.; Mills, D.P. The reactivity of gallium(I) and indium(I) halides towards bipyridines, terpyridines, imino-substituted pyridines and bis(imino)acenaphthenes. New J. Chem. 2004, 28, 207–213. [Google Scholar] [CrossRef]
- Dong, W.; Shu-Feng, S.I.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P. Syntheses and crystal structures of two ion pair complexes, [Ru(bpy)3]2[Fe(CN)6]I···7H2O and [Ru(bpy)3][Fe(CN)5NO]CH3OH···H2O. J. Coord. Chem. 2003, 56, 531–538. [Google Scholar] [CrossRef]
- Dong, W.; Ou-Yang, Y.; Song, H.-B.; Liao, D.-Z.; Jiang, Z.-H.; Yan, S.-P.; Cheng, P. Structure of an I–·(H2O)6 anion cluster in a 3D anion crystal host [I·(H2O)6Fe(CN)6·H2O]4. Inorg. Chem. 2006, 45, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T. Hydrogen-bond distances to halide ions in organic and organometallic crystal structures: Up-to-date database study. Acta Crystallogr. 1998, 54B, 456–463. [Google Scholar] [CrossRef]
- Marcos, V.; Stephens, A.J.; Jaramillo-Garcia, J.; Nussbaumer, A.L.; Woltering, S.L.; Valero, A.; Lemonnier, J.F.; Vitorica-Yrezabal, I.J.; Leigh, D.A. Allosteric initiation and regulation of catalysis with a molecular knot. Science 2016, 352, 1555–1559. [Google Scholar] [CrossRef] [Green Version]
- Hasenknopf, B.; Lehn, J.-M.; Kneisel, B.O.; Baum, G.; Fenske, D. Self-assembly of a circular double helicate. Angew. Chem. Int. Ed. Engl. 1996, 35, 1838–1840. [Google Scholar] [CrossRef]
- Danon, J.J.; Krüger, A.; Leigh, D.A.; Lemonnier, J.F.; Stephens, A.J.; Vitorica-Yrezabal, I.J.; Woltering, S.L. Braiding a molecular knot with eight crossings. Science 2017, 355, 159–162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Stephens, A.J.; Lemonnier, J.F.; Pirvu, L.; Vitorica-Yrezabal, I.J.; Robinson, C.J.; Leigh, D.A. Coordination chemistry of a molecular pentafoil knot. J. Am. Chem. Soc. 2019, 141, 3952–3958. [Google Scholar] [CrossRef] [Green Version]
- Sohncke, L. Entwickelung Einer Theorie der Krystallstruktur; B.G. Teubner: Leipzig, Germany, 1879. [Google Scholar]








| Halide | Number of Hits | Number of Hits Containing a 2,2′-bipyridine Ligand | Number of Hits for [M(bpy)3]n+ Complexes |
|---|---|---|---|
| F | 7 | 5 | 0 |
| Cl | 643 | 270 | 47 |
| Br | 126 | 46 | 9 |
| I | 105 | 53 | 8 |
| REFCODE Space Group | X M | H…X/Å | Mean H…X/Å | C…X/Å | Mean C…X/Å | C–H…X/° | Mean C–H…X/° | Ref |
|---|---|---|---|---|---|---|---|---|
| BUDKUG No. 5 | Cl Fe | 2.746–3.057 | 2.849 | 3.809–4.145 | 3.928 | 165.19–179.21 | 172.32 | [80,94] |
| BPNTAR No. 5 | Cl Ni | 2.706–2.9784 | 2.857 | 3.7663–4.0668 | 3.937 | 164.39–178.41 | 172.8 | [95] |
| BUDLIV No. 5 | Cl Co | 2.573–3.236 | 2.904 | 3.596–4.304 | 3.950 | 153.90–172.82 | 162.43 | [80] |
| CAMHED No. 179 | Cl Co | 2.525–2.920 | 2.696 | 3.610–3.989 | 3.771 | 164.97–174.56 | 169.45 | [96] |
| EFOWAA No. 5 | Cl Ni | 2.666–3.074 | 2.819 | 3.729–4.157 | 3.893 | 159.7–177.2 | 169.85 | [97] |
| EGOVEB No. 15 | Cl Ni | 2.777–2.892 | 2.844 | 3.856–3.977 | 3.925 | 170.4–174.6 | 172.0 | [90] |
| IPIMAW No. 5 | Cl Co | 2.699–2.870 | 2.809 | 3.767–3.955 | 3.888 | 166.9–176.3 | 172.1 | [98] |
| HUKWER No. 192 | Cl Ni | 2.9045 | 2.9045 | 3.986 | 3.986 | 172.0 | 172.0 | [99] |
| TIMSOY No. 17 | Cl Ni | 2.736–3.72 | 3.024 | 3.68–4.75 | 4.06 | 144.9–178.6 | 162.5 | [93] |
| XUJQEA * No. 2 | Cl Cu | 2.742–2.974 | 2.845 | 3.828–4.06 | 3.927 | 167.9–177.8 | 173.0 | [100] |
| CIBDOH No. 190 | Br Co | 2.8444–2.8935 | 2.8689 | 3.929–3.982 | 3.955 | 174.2–178.1 | 176.15 | [101] |
| IFAFUR No. 190 | Br Fe | 2.8118–3.0301 | 2.92095 | 3.88–4.12 | 4.00 | 168.4–175.8 | 172.1 | [91] |
| UBIWEK No. 54 | Br Fe | 2.777–2.9177 | 2.8439 | 3.854–4.006 | 3.921 | 167.2–177.8 | 170.9 | [102] |
| UBIXEL No. 54 | Br Ni | 2.7900–2.9307 | 2.8567 | 3.866–4.018 | 3.934 | 167.1–176.4 | 170.85 | [102] |
| UBIXIP No. 54 | Br Co | 2.7860–2.9492 | 2.8562 | 3.853–4.037 | 3.932 | 165.3–176.8 | 170.4 | [102] |
| UBIXOV No. 54 | Br Zn | 2.8000–2.9603 | 2.882 | 3.878–4.048 | 3.959 | 166.7–177.0 | 170.7 | [102] |
| BUCNUK No. 18 | I Ru | 2.936–3.205 | 3.021 | 3.99–4.29 | 4.09 | 160–178 | 170 | [103] |
| DUYBIK † No. 5 | I Ni | 2.899–3.219 3.874–4.585 (H-4) 3.176–3.618 | 3.083 4.247 (H-4) 3.421 | 3.797–3.972 4.864–5.538 (C-4) 3.909–4.127 | 3.885 5.227 (C-4) 4.037 | 125.8–141.8 148.9–155.0 (C-4) 112.8–127.7 | 133.4 152.2 (C-4) 120.6 | [89] |
| REXVOF No. 15 | I Li | 2.947–3.504 | 3.179 | 3.966–4.560 | 4.235 | 155.9–175.1 | 165.4 | [92] |
| REFCODE Metal Space Group | H…Cl/Å | Mean H…Cl/Å | C…Cl/Å | Mean C…Cl/Å | C–H…Cl/° | Mean C–H…Cl/° | Ref |
|---|---|---|---|---|---|---|---|
| DIWGIZ Rh No. 62 | 2.516–3.108 | 2.717 | 3.54–4.18 | 3.753 | 151.2–169.5 | 160.5 | [104] |
| CAMHIH Co No. 33 | 2.536–3.119 | 2.752 | 3.605–4.078 | 3.795 | 147.21–178.60 | 164.60 | [96] |
| POMHAB Co No. 64 | 2.463–2.9762 | 2.623 | 3.478–4.047 | 3.692 | 161.1–178.5 | 169.1 | [105] |
| POMHAB01 Co No. 64 | 2.4134–2.9391 | 2.601 | 3.463–4.013 | 3.672 | 161.4–178.2 | 169.5 | [106] |
| REFCODE Space Group | X M | H-6…X/Å | Mean H–6…X/Å | H–5…X/Å | Mean H–5…X/Å | Ref |
|---|---|---|---|---|---|---|
| HIGZAY No. 5 | Cl Ru | 3.132–3.497 | 3.320 | 3.327–4.217 | 3.669 | [107] |
| HIRDOB No. 5 | Cl Os | 3.061–3.590 | 3.304 | 3.353–4.110 | 3.700 | [81] |
| HIRDOB01 *,† No. 15 | Cl Os | 3.050–3.749 | 3.421 | 3.484–4.320 | 3.743 | [108] |
| HIRFAP01 No. 5 | Cl Ru | 3.145–3.578 | 3.376 | 3.312–4.249 | 3.664 | [109] |
| INIYIN No. 15 | Cl Ru | 3.140–3.712 | 3.408 | 3.407–4.216 | 3.696 | [110] |
| HIRDUH01 No. 5 | Br Ru | 3.189–3.552 | 3.430 | 3.323–4.091 | 3.678 | [109] |
| ISIMOM No. 33 | I Ga | 3.171–3.443 | 3.326 | 3.796–4.200 | 3.942 | [111] |
| TAHNOG No. 9 | I Ru | 3.141–4.163 | 3.641 | 3.383–4.980 | 3.984 | [112] |
| TAHNOG01 No. 9 | I Ru | 3.108–4.1762 | 3.631 | 3.2959–4.908 | 3.923 | [113] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constable, E.C.; Housecroft, C.E. Halide Ion Embraces in Tris(2,2′-bipyridine)metal Complexes. Crystals 2020, 10, 671. https://doi.org/10.3390/cryst10080671
Constable EC, Housecroft CE. Halide Ion Embraces in Tris(2,2′-bipyridine)metal Complexes. Crystals. 2020; 10(8):671. https://doi.org/10.3390/cryst10080671
Chicago/Turabian StyleConstable, Edwin C., and Catherine E. Housecroft. 2020. "Halide Ion Embraces in Tris(2,2′-bipyridine)metal Complexes" Crystals 10, no. 8: 671. https://doi.org/10.3390/cryst10080671
APA StyleConstable, E. C., & Housecroft, C. E. (2020). Halide Ion Embraces in Tris(2,2′-bipyridine)metal Complexes. Crystals, 10(8), 671. https://doi.org/10.3390/cryst10080671