Optical and Geometrical Characterizations of Non-Linear Supramolecular Liquid Crystal Complexes
Abstract
:1. Introduction
2. Experimental
Preparation of 1:1 Supramolecular Complexes
3. Results and Discussion
3.1. FT-IR Spectroscopic Confirmation of SMHB Complex Formation
3.2. Mesomorphic Properties
3.3. DFT Calculations
3.4. Frontier Molecular Orbitals and Molecular Electrostatic Potential
3.5. Refractive Index Measurements
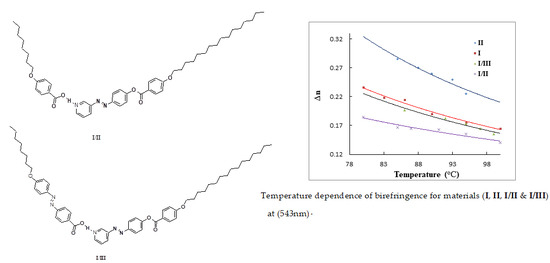
3.6. Birefringence Measurements
3.6.1. Measuring Birefringence by Using Abbe Refractometer
3.6.2. Measuring Birefringence by Using Modified Spectrophotometer
3.7. Order Parameters Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- De Gennes, P.-G.; Prost, J. The Physics of Liquid Crystals; Oxford University Press: Oxford, UK, 1993; Volume 83. [Google Scholar]
- Chandrasekhar, S. Liquid Crystals; Cambridge University Press: Cambridge, UK; New York, NY, USA, 1994; Volume 49, pp. 587–588. [Google Scholar]
- Priestly, E. Introduction to Liquid Crystals; Springer: Berlin, Germany, 2012. [Google Scholar]
- Aleksandriiskii, V.; Novikov, I.; Kuvshinova, S.; Burmistrov, V.; Koifman, O. Dielectric, optical and orientational properties of liquid crystalline 4-alkyloxy-4′-cyanoazoxybenzenes and 4-alkyloxy-4′-cyanoazobenzenes. J. Mol. Liq. 2016, 223, 1270–1276. [Google Scholar] [CrossRef]
- Gray, G.W.; Vill, V.; Spiess, H.W.; Demus, D.; Goodby, J.W. Physical Properties of Liquid Crystals; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Wilhelm, E.; Letcher, T. Heat Capacities: Liquids, Solutions and Vapours; Royal Society of Chemistry: London, UK, 2010. [Google Scholar]
- Nalwa, H.S. Handbook of Low and High Dielectric Constant Materials and Their Applications, Two-Volume Set; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar]
- Kumar, S. Experimental Study of Physical Properties and Phase Transitions; Cambridge University Press: New York, NY, USA, 2001. [Google Scholar]
- Gramsbergen, E.F.; de Jeu, W.H. First-and second-order smectic-A to nematic phase transitions in p, p′-dialkylazoxybenzenes studied by birefringence. J. Chem. Soc. Faraday Trans. 2 Mol. Chem. Phys. 1988, 84, 1015–1021. [Google Scholar] [CrossRef]
- Nesrullajev, A. Texture transformations and thermo-optical properties of nematic mesogen at nematic–isotropic liquid phase transition. J. Mol. Liq. 2014, 196, 217–222. [Google Scholar] [CrossRef]
- Zaki, A. Optical measurements of mixture thermotropic liquid crystals. Opt. Lasers Eng. 2010, 48, 538–542. [Google Scholar] [CrossRef]
- El-Dessouki, T.A.; Roushdy, M.; Hendawy, N.I.; Naoum, M.M.; Zaki, A.A. Optical measurements of thermotropic liquid crystals. J. Mod. Phys. 2013, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Zaki, A.A. Optical measurements and Speckle Photography for thermotropic liquid crystals Mixtures. J. Mod. Phys. 2013, 4, 517. [Google Scholar] [CrossRef] [Green Version]
- Miszczykd, E.; Pieceka, W. Application of modified interference wedge method in measurements of indices of refraction and birefringence of nematic liquid crystals. Acta Phys. Pol. A 2013, 124, 946–948. [Google Scholar]
- Okumuş, M.; Özgan, Ş.; Kırık, İ.; Kerli, S. Thermal and optical characterization of liquid crystal 4′-hexyl-4-biphenylcarbonitrile/4-hexylbenzoic acid mixtures. J. Mol. Struct. 2016, 1120, 150–155. [Google Scholar] [CrossRef]
- Devi, T.; Choudhury, B.; Bhattacharjee, A.; Dabrowski, R. Study of optical parameters of two fluorinated isothiocyanato nematic liquid crystals exhibiting high birefringence. Opto-Electron. Rev. 2014, 22, 24–30. [Google Scholar] [CrossRef]
- Thingujam, K.; Bhattacharjee, A.; Choudhury, B.; Dabrowski, R. Nematic liquid crystals exhibiting high birefringence. Opt. Rev. 2016, 23, 409–419. [Google Scholar] [CrossRef]
- Dąbrowski, R.; Kula, P.; Herman, J. High birefringence liquid crystals. Crystals 2013, 3, 443–482. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Zaki, A. Optical measurements of phase transitions in difluorophenylazophenyl benzoate thermotropic liquid crystal with specific orientated fluorine atoms. Phase Transit. 2019, 92, 135–148. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, X.; Lu, H.; Su, Z.; Zhou, Y.; Song, B.; Li, X.; Yang, X.; Tu, Y.; Li, C.Y. Effect of Fullerene Volume Fraction on Two-Dimensional Crystal-Constructed Supramolecular Liquid Crystals. Chem. Asian J. 2019, 14, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Dechant, M.; Gerbig, L.; Baumann, M. Supramolecular click procedures in liquid crystals. Liq. Cryst. 2019, 46, 1985–1994. [Google Scholar] [CrossRef]
- Saccone, M.; Pfletscher, M.; Kather, S.; Wölper, C.; Daniliuc, C.; Mezger, M.; Giese, M. Improving the mesomorphic behaviour of supramolecular liquid crystals by resonance-assisted hydrogen bonding. J. Mater. Chem. C 2019, 7, 8643–8648. [Google Scholar] [CrossRef]
- Sharma, V.S.; Shah, A.P.; Sharma, A.S. A new class of supramolecular liquid crystals derived from azo calix [4] arene functionalized 1, 3, 4-thiadiazole derivatives. New J. Chem. 2019, 43, 3556–3564. [Google Scholar] [CrossRef]
- Wang, X.; Bai, L.; Kong, S.; Song, Y.; Meng, F. Star-shaped supramolecular ionic liquid crystals based on pyridinium salts. Liq. Cryst. 2019, 46, 512–522. [Google Scholar] [CrossRef]
- Alhaddad, O.; Ahmed, H.; Hagar, M. Experimental and theoretical approaches of new nematogenic chair architectures of supramolecular H-bonded liquid crystals. Molecules 2020, 25, 365. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Hagar, M.; Alhaddad, O. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. RSC Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef] [Green Version]
- Nafee, S.S.; Ahmed, H.A.; Hagar, M. New architectures of supramolecular H-bonded liquid crystal complexes based on dipyridine derivatives. Liq. Cryst. 2020, 1–14. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Ahmed, H.A.; Hagar, M.; Abu Al-Ola, K.A.; Alrefay, B.S.; Haddad, B.A.; Albalawi, R.F.; Aljuhani, R.H.; Aloqebi, L.D.; Alsenani, S.F. Induced phases of new H-bonded supramolecular liquid crystal complexes; mesomorphic and geometrical estimation. Molecules 2020, 25, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnoman, R.B.; Hagar, M.; Ahmed, H.A.; Abu Al-Ola, K.A.; Naoum, M.M.; Al-Elati, F.; Abdullah Zaid, Y.; Alsharif, A.; Al-Juhani, Y.; Abulrhelh, A. Characterization of New H-Bonded Liquid Crystalline Complexes Based on Iminophenyl Nicotinate. Crystals 2020, 10, 499. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Abu Al-Ola, K.A.; Hagar, M.; Ahmed, H.A. Chair-and V-Shaped of H-bonded Supramolecular Complexes of Azophenyl Nicotinate Derivatives; Mesomorphic and DFT Molecular Geometry Aspects. Molecules 2020, 25, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, G.W.; Jones, B. The mesomorphic transition points of the para-normal-alkoxybenzoic acids-a correction. J. Chem. Soc. 1953, 4179–4180. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Kato, T.; Wilson, P.G.; Fujishima, A.; Fréchet, J.M. Hydrogen-bonded liquid crystals. A novel mesogen incorporating nonmesogenic 4, 4′-bipyridine through selective recognition between hydrogen bonding donor and acceptor. Chem. Lett. 1990, 19, 2003–2006. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M.; Wilson, P.G.; Saito, T.; Uryu, T.; Fujishima, A.; Jin, C.; Kaneuchi, F. Hydrogen-bonded liquid crystals. Novel mesogens incorporating nonmesogenic bipyridyl compounds through complexation between hydrogen-bond donor and acceptor moieties. Chem. Mater. 1993, 5, 1094–1100. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, J.M.J. Hydrogen bonding and the self-assembly of supramolecular liquid-crystalline materials. Macromol. Symp. 1995, 98, 311–326. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Frechet, J.M. Supramolecular liquid-crystalline networks built by self-assembly of multifunctional hydrogen-bonding molecules. Chem. Mater. 1996, 8, 961–968. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Frechet, J.M. Induction of a cholesteric phase via self-assembly in supramolecular networks built of non-mesomorphic molecular components. Liq. Cryst. 1998, 24, 413–418. [Google Scholar] [CrossRef]
- Chen, K.-Y. Crystal Structure, Hydrogen-Bonding Properties, and DFT Studies of 2-((2-(2-Hydroxyphenyl) benzo [d] thiazol-6-yl) methylene) malononitrile. Mol. Cryst. Liq. Cryst. 2015, 623, 285–296. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Reig, M.; Cuadrado, A.; Ortega, M.; Nonell, S.; Velasco, D. A photoswitchable bis-azo derivative with a high temporal resolution. Chem. Commun. 2014, 50, 11462–11464. [Google Scholar] [CrossRef] [PubMed]
- Shoji, M.; Tanaka, F. Theoretical study of hydrogen-bonded supramolecular liquid crystals. Macromolecules 2002, 35, 7460–7472. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT Calculations and Mesophase Study of Coumarin Esters and Its Azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef] [Green Version]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Quinazolin-4-yl-sulfanylacetyl-hydrazone derivatives; Synthesis, molecular structure and electronic properties. J. Mol. Struct. 2013, 1049, 177–188. [Google Scholar] [CrossRef]
- Soliman, S.M.; Hagar, M.; Ibid, F.; El Sayed, H. Experimental and theoretical spectroscopic studies, HOMO–LUMO, NBO analyses and thione–thiol tautomerism of a new hybrid of 1, 3, 4-oxadiazole-thione with quinazolin-4-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 270–279. [Google Scholar] [CrossRef]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4 (3H)-one derivatives; an account on the N-versus S-Alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Hagar, M.; Soliman, S.M. Ultrasonic Synthesis, Molecular Structure and Mechanistic Study of 1, 3-Dipolar Cycloaddition Reaction of 1-Alkynylpyridinium-3-olate and Acetylene Derivatives. Molecules 2016, 21, 848. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff base/ester liquid crystals with different lateral substituents: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Paterson, D.A.; Gao, M.; Kim, Y.-K.; Jamali, A.; Finley, K.L.; Robles-Hernández, B.; Diez-Berart, S.; Salud, J.; de la Fuente, M.R.; Timimi, B.A. Understanding the twist-bend nematic phase: The characterisation of 1-(4-cyanobiphenyl-4′-yloxy)-6-(4-cyanobiphenyl-4′-yl) hexane (CB6OCB) and comparison with CB7CB. Soft Matter 2016, 12, 6827–6840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; An, Z.; Wang, W.; Chen, X.; Chen, P. Lateral substituent effects on UV stability of high-birefringence liquid crystals with the diaryl-diacetylene core: DFT/TD-DFT study. Liq. Cryst. 2017, 44, 1515–1524. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: Mesophase behaviour and DFT calculations. Liq. Cryst. 2020, 47, 114–124. [Google Scholar] [CrossRef]
- Nafee, S.S.; Ahmed, H.; Hagar, M. Theoretical, experimental and optical study of new thiophene-based liquid crystals and their positional isomers. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef] [Green Version]
- Alnoman, R.; Ahmed, H.A.; Hagar, M. Synthesis, optical, and geometrical approaches of new natural fatty acids’ esters/Schiff base liquid crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, H.; Hagar, M.; Alhaddad, O. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2019, 47, 404–413. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Ahmed, H.A.; Hagar, M.; Saad, G.R.; Abu Al-Ola, K.A.; Naoum, M.M. Thermal and photophysical studies of binary mixtures of liquid crystal with different geometrical mesogens. Crystals 2020, 10, 223. [Google Scholar] [CrossRef] [Green Version]
- Alnoman, R.B.; Hagar, M.; Ahmed, H.A.; Naoum, M.M.; Sobaih, H.A.; Almshaly, J.S.; Haddad, M.M.; Alhaisoni, R.A.; Alsobhi, T.A. Binary Liquid Crystal Mixtures Based on Schiff Base Derivatives with Oriented Lateral Substituents. Crystals 2020, 10, 319. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Aljohani, G.; Alhaddad, O.A. Investigation of Some Antiviral N-Heterocycles as COVID 19 Drug: Molecular Docking and DFT Calculations. Int. J. Mol. Sci. 2020, 21, 3922. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.A.; Mansour, E.; Hagar, M. Mesomorphic study and DFT simulation of calamitic Schiff base liquid crystals with electronically different terminal groups and their binary mixtures. Liq. Cryst. 2020, 1–13. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Aouad, M.R. Mesomorphic and DFT diversity of Schiff base derivatives bearing protruded methoxy groups. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4′′-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Refaie, A.A.; Alaasar, M.A. Novel hydrogen-bonded angular supramolecular liquid crystals. Liq. Cryst. 2012, 39, 47–61. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2016, 43, 222–234. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2018, 46, 550–559. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. New azobenzene-based natural fatty acid liquid crystals with low melting point: Synthesis, DFT calculations and binary mixtures. Liq. Cryst. 2019, 46, 2223–2234. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. Rsc. Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef] [Green Version]
- Cleland, W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Lizu, M.; Lutfor, M.; Surugau, N.; How, S.; Arshad, S.E. Synthesis and characterization of ethyl cellulose–based liquid crystals containing azobenzene chromophores. Mol. Cryst. Liq. Cryst. 2010, 528, 64–73. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Ghanem, A.; Noel, C. FTIR investigation of two alkyl-p-terphenyl-4, 4″-dicarboxylates in their crystalline, smectic and isotropic phases. Mol. Cryst. Liq. Cryst. 1987, 150, 447–472. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; Marcelis, A.T.; Storey, J.M.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.; Martinez-Felipe, A.; Paterson, D.; Forsyth, E.; Lawrence, G.; Henderson, P.; Storey, J.; Gorecka, E. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1: 1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino) phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, G.; Zhong, X.; Yua, Y.; Ganb, W. Effect of pi–pi stacking on the self-assembly of azomethine-type rod–coil liquid crystals. Liq. Cryst. 2011, 38, 995–1006. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Saad, G. Impact of the proportionation of dialkoxy chain length on the mesophase behaviour of Schiff base/ester liquid crystals; experimental and theoretical study. Liq. Cryst. 2019, 46, 1611–1620. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; El-Sayed, T.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Sreeramakavacham, S.S.; Rao, B.G.S.; Mallika, K.; Kumari, T.V.; Lakshminarayana, S.; Ha, S.T. Novel method for order parameter of ferroelectric liquid crystals by image analysis. Liq. Cryst. 2013, 40, 384–390. [Google Scholar] [CrossRef]
- Wu, S.-T.; Hsu, C.-S.; Shyu, K.-F. High birefringence and wide nematic range bis-tolane liquid crystals. Appl. Phys. Lett. 1999, 74, 344–346. [Google Scholar] [CrossRef] [Green Version]
- Haller, I. Thermodynamic and static properties of liquid crystals. Prog. Solid State Chem. 1975, 10, 103–118. [Google Scholar] [CrossRef]
- Patari, S.; Chakraborty, S.; Nath, A. The optical anisotropy and orientational order parameter of two mesogens having slightly different flexible side chain–a comparative study. Liq. Cryst. 2016, 43, 1017–1027. [Google Scholar] [CrossRef]
- Sastry, S.S.; Kumari, T.V.; Mallika, K.; Rao, B.G.S.; Ha, S.-T.; Lakshminarayana, S. Order parameter studies on EPAP alkanoate mesogens. Liq. Cryst. 2012, 39, 295–301. [Google Scholar] [CrossRef]
- Kumar, A. Determination of orientational order and effective geometry parameter from refractive indices of some nematics. Liq. Cryst. 2013, 40, 503–510. [Google Scholar] [CrossRef]
- Erkan, S.; Cetinkaya, M.; Yildiz, S.; Özbek, H. Critical behavior of a nonpolar smectogen from high-resolution birefringence measurements. Phys. Rev. E 2012, 86, 041705. [Google Scholar] [CrossRef]
- Li, J.; Wen, C.-H.; Gauza, S.; Lu, R.; Wu, S.-T. Refractive indices of liquid crystals for display applications. J. Disp. Technol. 2005, 1, 51. [Google Scholar] [CrossRef]


















| Compound | ύOH (cm−1) | O-H (Å) | ύC=O (cm−1) | C=O (Å) | ύC=NPyr (cm−1) | C=NPyr (Å) | ύNr=NPyr (cm−1) | N=NPyr (Å) | ύH-bond (cm−1) | H-Bond (Å) |
|---|---|---|---|---|---|---|---|---|---|---|
| I | - | - | - | - | 1615.9 | 1.347 | 1475.6 | 1.211 | - | - |
| II | 3662.6 | 0.976 | 1691.9 | 1.238 | - | - | - | - | - | - |
| III | 3660.7 | 0.976 | 1691.1 | 1.237 | - | - | 1475.8 | 1.282 | - | - |
| I/II | 2561.3 | 1.032 | 1668.5 | 1.251 | 1617.7 | 1.348 | 1475.5 | 1.281 | 2561.3 | 1.619 |
| I/III | 2469.9 | 1.038 | 1669.1 | 1.250 | 1618.2 | 1.348 | 1475.4 | 1.282 | 2469.9 | 1.597 |
| Sample | TCr-SmC | ∆HCr-SmC | TCr-SmA | ∆HCr-SmA | TSmC-N | ∆HSmC-N | TSmA-I | ∆HSmA-I | TN-I | ∆HN-I |
|---|---|---|---|---|---|---|---|---|---|---|
| I | - | - | 96.1 | 23.5 | - | - | 121.5 | 1.6 | - | - |
| II | 101.0 | 47.0 | - | - | 108.0 | 4.7 | - | - | 147.0 | 1.9 |
| III | 147.0 | 55.6 | - | - | 241.0 | 7.9 | - | - | 262.5 | 3.2 |
| I/II | - | 76.4 | 27.4 | - | 104.6 | 1.5 | - | - | ||
| I/III | 91.8 | 46.0 | - | - | 137.0 | 7.2 | - | - | 198.7 | 3.5 |
| Parameter | Ia | Ib | II | III | I/II | I/III |
|---|---|---|---|---|---|---|
| Ecorr | 0.74 | 0.73 | 0.35 | 0.44 | 1.08 | 1.17 |
| ZPVE | −1711.82 | −1711.82 | −810.17 | −1150.57 | −2522.02 | −2862.42 |
| Etot | −1711.78 | −1711.78 | −810.15 | −1150.54 | −2521.96 | −2862.35 |
| H | −1711.78 | −1711.78 | −810.15 | −1150.54 | −2521.96 | −2862.35 |
| G | −1711.91 | −1711.91 | −810.22 | −1150.63 | −2522.14 | −2862.55 |
| Total Dipole Moment | 5.2 | 7.7 | 4.3 | 5.3 | 4.6 | 4.2 |
| Polarizability α | 474.0 | 473.3 | 182.5 | 318.2 | 676.8 | 820.2 |
| Compound | EHOMO (Kcal/mol) | ELUMO (Kcal/mol) | ΔE(ELUMO − EHOMO) (Kcal/mol) | η= ΔE(ELUMO − EHOMO)/2 | S = 1/ΔE |
|---|---|---|---|---|---|
| I | −149.97 | −64.00 | 85.97 | 42.98 | 0.012 |
| II | −151.86 | −34.51 | 117.34 | 58.67 | 0.009 |
| III | −143.70 | −64.63 | 79.07 | 39.53 | 0.013 |
| I/II | −142.44 | −69.03 | 73.42 | 36.71 | 0.014 |
| I/III | −137.42 | −69.65 | 67.77 | 33.89 | 0.015 |
| Sample | Δn Temp. (100 °C) | Δno | β |
|---|---|---|---|
| I | 0.18 ± 0.05 | 0.35 | 0.12 |
| II | 0.23 ± 0.05 | 0.55 | 0.32 |
| I/II | 0.16 ± 0.05 | 0.21 | 0.02 |
| I/III | 0.17 ± 0.05 | 0.38 | 0.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.A.; Hagar, M.; Alhaddad, O.A.; Zaki, A.A. Optical and Geometrical Characterizations of Non-Linear Supramolecular Liquid Crystal Complexes. Crystals 2020, 10, 701. https://doi.org/10.3390/cryst10080701
Ahmed HA, Hagar M, Alhaddad OA, Zaki AA. Optical and Geometrical Characterizations of Non-Linear Supramolecular Liquid Crystal Complexes. Crystals. 2020; 10(8):701. https://doi.org/10.3390/cryst10080701
Chicago/Turabian StyleAhmed, Hoda A., Mohamed Hagar, Omaima A. Alhaddad, and Ayman A. Zaki. 2020. "Optical and Geometrical Characterizations of Non-Linear Supramolecular Liquid Crystal Complexes" Crystals 10, no. 8: 701. https://doi.org/10.3390/cryst10080701
APA StyleAhmed, H. A., Hagar, M., Alhaddad, O. A., & Zaki, A. A. (2020). Optical and Geometrical Characterizations of Non-Linear Supramolecular Liquid Crystal Complexes. Crystals, 10(8), 701. https://doi.org/10.3390/cryst10080701