Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching—Alkali Melting Activation and Hydrothermal Synthesis
Abstract
:1. Introduction
2. Experimental
2.1. Materials
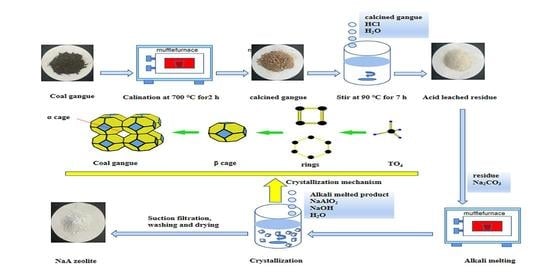
2.2. Technological Process
2.3. Characterization
3. Results and Discussions
3.1. Major Chemical Elements Analysis of Coal Gangue and the Acid Leached Residue
3.2. Phase Analysis of the Residue and Activation Product
3.3. Effects of n(SiO2)/n(Al2O3) on the Product Phase
3.4. Effects of n(Na2O)/n(SiO2) on the Phase
3.5. Effects of n(H2O)/n(Na2O) on the Phase
3.6. Effect of Aging Temperature on the Phase
3.7. Effect of Crystallization Time on the Phase and Morphology
3.8. XRD and SEM Analyses of the Product under Optimized Conditions
3.9. Preparation Mechanism
4. Conclusions
- (1)
- Calcination of the coal gangue at 700 °C for 2 h and then leaching the calcined powder by 20% hydrochloric acid; the liquid to solid ratio is 3:1 at 90 °C for 7 h and can remove most of the iron ions.
- (2)
- Evenly mixing the sodium carbonate with the acid-leached filter residue according to the mass ratio of m(acid leached residue): m(sodium carbonate) = 1:1.1, the mixture is melted at 750 °C for 2 h, then acid-leached filter residue turns into NaAlSiO4; the rests are amorphous SiO2 and Al2O3, which both have high chemical activity and can participate in the crystallization reaction, so the acid-leached filter residue is activated absolutely.
- (3)
- n(SiO2)/n(Al2O3) = 2.0, n(Na2O)/n(SiO2) = 2.1, n(H2O)/n(Na2O) = 55, aging at 60 °C for 2 h, and crystallization at 94 °C for 4 h are the optimized conditions for NaA zeolite synthesis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, X.; Guo, S.; Gao, L.; Cao, Y.; Wei, X. Mercury release during thermal treatment of two coal gangues and two coal slimes under N2 and in air. Energy Fuels 2017, 31, 8648–8654. [Google Scholar] [CrossRef]
- Xu, H.; Song, W.; Cao, W.; Gang, S.; Lu, H.; Yang, D.; Chen, D.; Zhang, R. Utilization of coal gangue for the production of brick. J. Mater. Cycles Waste Manag. 2016, 19, 1270–1278. [Google Scholar] [CrossRef]
- Du, H.; Ma, L.; Liu, X.-Y.; Zhang, F.; Yang, X.; Wu, Y.; Zhang, J. A novel mesoporous SiO2 material with MCM-41 structure from coal gangue: Preparation, ethylenediamine modification, and adsorption properties for CO2 capture. Energy Fuels 2018, 32, 5374–5385. [Google Scholar] [CrossRef]
- Liu, M.; Zhu, Z.; Zhang, Z.; Chu, Y.; Yuan, B.; Wei, Z. Development of highly porous mullite whisker ceramic membranes for oil-in-water separation and resource utilization of coal gangue. Sep. Purif. Technol. 2020, 237, 116483. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Y.; Duan, X. Study on the factors affecting the deep reduction of coal gangue containing high contents of iron and sulfur. Fuel 2020, 288, 119571. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, H.; Wu, C.; Chen, H.; Sun, J.; Liu, J. Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technol. 2020, 368, 112–124. [Google Scholar] [CrossRef]
- Xiao, M.; Ju, F.; He, Z. Research on shotcrete in mine using non-activated waste coal gangue aggregate. J. Clean. Prod. 2020, 259, 120810. [Google Scholar] [CrossRef]
- Du, T.; Wang, D.; Bai, Y.; Zhang, Z. Optimizing the formulation of coal gangue planting substrate using wastes: The sustainability of coal mine ecological restoration. Ecol. Eng. 2020, 143, 105669. [Google Scholar] [CrossRef]
- Jouni, R.; Katja, O.; Paivo, K.; Marcello, R.; Mirja, I. Milling of peat-wood fly ash: Effect on water demand of mortar and rheology of cement paste. Constr. Build. Mater. 2018, 180, 143–153. [Google Scholar]
- Zhou, C.; Liu, G.; Wu, S.; Lam, P.K.S. The environmental characteristics of usage of coal gangue in bricking-making: A case study at Huainan, China. Chemosphere 2014, 95, 274–280. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Y.; Liu, L.; Seshadri, S.; Wang, X.; Zhang, Z. Integrated utilization of sewage sludge and coal gangue for cement clinker products: Promoting tricalcium silicate formation and trace elements immobilization. Materials 2016, 9, 275. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Jin, X.; Song, C.; Bi, Y.; Liu, Q.; Liu, C.; Ji, N.; Lu, X.; Ma, D.; Li, Z. Rapid synthesis and NH3-SCR activity of SSZ-13 zeolite via coal gangue. Green Chem. 2019, 22, 219–229. [Google Scholar] [CrossRef]
- Chen, J.; Lu, X. Synthesis and characterization of zeolites NaA and NaX from coal gangue. J. Mater. Cycles Waste Manag. 2017, 20, 489–495. [Google Scholar] [CrossRef]
- Jin, Y.; Li, L.; Liu, Z.; Zhu, S.; Wang, D. Synthesis and characterization of low-cost zeolite NaA from coal gangue by hydrothermal method. Adv. Powder Technol. 2021, 32, 791–801. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y. Synthesis and characterization of zeolite A obtained from coal gangue for the adsorption of F– in wastewater. Sci. Adv. Mater. 2019, 11, 277–282. [Google Scholar] [CrossRef]
- Ren, J.; Xie, C.; Guo, X.; Qin, Z.; Lin, J.; Li, Z. Combustion characteristics of coal gangue under an atmosphere of coal mine methane. Energy Fuels 2012, 28, 3688–3695. [Google Scholar] [CrossRef]
- Merrikhpour, H.; Jalali, M. Comparative and competitive adsorption of cadmium, copper, nickel, and lead ions by iranian natural zeolite. Clean. Technol. Environ. Policy 2013, 15, 303–316. [Google Scholar] [CrossRef]
- Polat, E.; Karaca, M.; Demir, H.; Onus, A.N. Use of natural zeolite (clinoptilolite) in agriculture. J. Fruit Ornam. Plant Res. 2004, 12, 183–189. [Google Scholar]
- Muzic, M.; Sertic-Bionda, K.; Adzamic, T. Evaluation of commercial adsorbents and their application for desulfurization of model fuel. Clean. Technol. Environ. Policy 2012, 14, 283–290. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Xiao, F. Mesoporous zeolites as efficient catalysts for oil refining and natural gas conversion. Front. Chem. Sci. Eng. 2013, 7, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Müller, U. Catalytic applications of zeolites in chemical industry. Top. Catal. 2009, 52, 888–895. [Google Scholar] [CrossRef] [Green Version]
- Qian, T.; Li, J. Synthesis of Na-A zeolite from coal gangue with the in-situ crystallization technique. Adv. Powder Technol. 2015, 26, 98–104. [Google Scholar] [CrossRef]
- Lu, X.; Shi, D.; Chen, J. Sorption of Cu2+ and Co2+ using zeolite synthesized from coal gangue: Isotherm and kinetic studies. Environ. Earth Sci. 2017, 76, 591. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Wang, J.; Zhou, J.; Huang, X.; Chen, L.; Hu, P.; Gao, J.; Zhen, Q.; Bashir, S.; et al. Facile preparation of zeolite-activated carbon composite from coal gangue with enhanced adsorption performance. Chem. Eng. J. 2020, 390, 124513. [Google Scholar] [CrossRef]
- Jha, B.; Singh, D.N. A three step process for purification of fly ash zeolites by hydrothermal treatment. Appl. Clay Sci. 2014, 90, 122–129. [Google Scholar] [CrossRef]
- Khaleque, A.; Alam, M.; Hoque, M.; Mondal, S.; Bin Haider, J.; Xu, B.; Johir, M.; Karmakar, A.K.; Zhou, J.; Ahmed, M.B.; et al. Zeolite synthesis from low-cost materials and environmental applications: A review. Environ. Adv. 2020, 2, 100019. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Niceforo, G.; Lettino, A. Sodalite, faujasite and A-type zeolite from 2:1dioctahedral and 2:1:1 trioctahedral clay minerals. A singular review of synthesis methods through laboratory trials at a low incubation temperature. Powder Technol. 2017, 320, 483–497. [Google Scholar] [CrossRef]
- Ayele, L.; Perez-Pariente, J.; Chebude, Y.; Diaz, I. Conventional versus alkali fusion synthesis of zeolite A from low grade kaolin. Appl. Clay Sci. 2016, 132, 485–490. [Google Scholar] [CrossRef]
- Bi, H.; Wang, C.; Lin, Q.; Jiang, X.; Jiang, C.; Bao, L. Combustion behavior, kinetics, gas emission characteristics and artificial neural network modeling of coal gangue and biomass via TG-FTIR. Energy 2020, 213, 118790. [Google Scholar] [CrossRef]
- Han, J.; Ha, Y.; Guo, M.; Zhao, P.; Liu, Q.; Liu, C.; Song, C.; Ji, N.; Lu, X.; Ma, D.; et al. Synthesis of zeolite SSZ-13 from coal gangue via ultrasonic pretreatment combined with hydrothermal growth method. Ultrason. Sonochemistry 2019, 59, 104703. [Google Scholar] [CrossRef] [PubMed]
- Eilertsen, E.A.; Nilsen, M.H.; Wendelbo, R.; Olsbye, U.; Lillerud, K.P. Synthesis of high silica CHA zeolites with controlled Si/Al ratio. Stud. Surf. Sci. Catal. 2008, 174, 265–268. [Google Scholar]
- Wang, Q.; Wang, L.; Wang, H.; Li, Z.; Zhang, X.; Zhang, S.; Zhou, K. Effect of SiO2/Al2O3 ratio on the conversion of methanol to olefins over molecular sieve catalysts. Front. Chem. Sci. Eng. 2011, 5, 79–88. [Google Scholar] [CrossRef]
- Kosinov, N.; Auffret, C.; Borghuis, G.J.; Sripathi, V.; Hensen, E. Influence of the si/al ratio on the separation properties of SSZ-13 zeolite membranes. J. Membr. Sci. 2015, 484, 140–145. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Z.; Li, X.; Bao, S.; Liu, L.; He, H.; Lai, X.; Zhang, L.; Zhang, P. Preparation of ZSM-5 molecular sieve by diatomaceous earth in-situ crystallization. Integr. Ferroelectr. 2018, 189, 165–174. [Google Scholar] [CrossRef]
- Yao, G.; Lei, J.; Zhang, X.; Sun, Z.; Zheng, S. One-step hydrothermal synthesis of zeolite X powder from natural low-grade diatomite. Materials 2018, 11, 906. [Google Scholar] [CrossRef] [Green Version]
- Maia, A.Á.B.; Dias, R.N.; Angélica, R.S.; Neves, R.F. Influence of an aging step on the synthesis of zeolite NaA from brazilian amazon kaolin waste. J. Mater. Res. Technol. 2019, 8, 2924–2929. [Google Scholar] [CrossRef]
- Guo, Y.P.; Lee, N.H.; Oh, H.-J.; Yoon, C.R.; Rhee, C.K.; Lee, K.S.; Kim, S.J. Fabrication of oriented TiO2 based nanotube array thin films. Solid State Phenom. 2008, 135, 19–22. [Google Scholar] [CrossRef]
- Behin, J.; Bukhari, S.S.; Kazemian, H.; Rohan, S. Developing a zero liquid discharge process for zeolitization of coal fly ash to synthetic NaP zeolite. Fuel 2016, 7, 195–202. [Google Scholar] [CrossRef]
- Bukhari, S.S.; Behin, J.; Kazemian, H.; Rohani, S. A comparative study using direct hydrothermal and indirect fusion methods to produce zeolites from coal fly ash utilizing single-mode microwave energy. J. Mater. Sci. Lett. 2014, 49, 8261–8271. [Google Scholar] [CrossRef]
- Bu, N.; Liu, X.; Song, S.; Liu, J.; Yang, Q.; Li, R.; Zheng, F.; Yan, L.; Zhen, Q.; Zhang, J. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution. Adv. Powder Technol. 2020, 31, 2699–2710. [Google Scholar] [CrossRef]
- Li, Y.; Peng, T.; Man, W.; Ju, L.; Zheng, F.; Zhang, M.; Guo, M. Hydrothermal synthesis of mixtures of NaA zeolite and sodalite from Ti-bearing electric arc furnace slag. RSC Adv. 2016, 6, 8358–8366. [Google Scholar] [CrossRef]
- Su, Q.; He, Y.; Yang, S.; Wan, H.; Cui, X. Synthesis of NaA-zeolite microspheres by conversion of geopolymer and their performance of Pb (II) removal. Appl. Clay Sci. 2020, 20, 105914. [Google Scholar] [CrossRef]



















| Compositions | Gangue | Residue |
|---|---|---|
| SiO2 | 42.18 | 83.12 |
| Al2O3 | 20.43 | 10.50 |
| Fe2O3 | 15.36 | 0.99 |
| K2O | 1.24 | 1.04 |
| Na2O | 0.40 | 0.38 |
| CaO | 2.95 | 0.04 |
| MgO | 1.61 | 0.19 |
| MgO | 1.66 | 2.77 |
| TiO2 | 0.25 | 0.04 |
| MnO | 0.54 | 0.03 |
| S | 0.30 | 0.06 |
| FC and other ignition loss | 13.08 | 0.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, D.; Jiang, R. Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching—Alkali Melting Activation and Hydrothermal Synthesis. Crystals 2021, 11, 1198. https://doi.org/10.3390/cryst11101198
Kong D, Jiang R. Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching—Alkali Melting Activation and Hydrothermal Synthesis. Crystals. 2021; 11(10):1198. https://doi.org/10.3390/cryst11101198
Chicago/Turabian StyleKong, Deshun, and Rongli Jiang. 2021. "Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching—Alkali Melting Activation and Hydrothermal Synthesis" Crystals 11, no. 10: 1198. https://doi.org/10.3390/cryst11101198
APA StyleKong, D., & Jiang, R. (2021). Preparation of NaA Zeolite from High Iron and Quartz Contents Coal Gangue by Acid Leaching—Alkali Melting Activation and Hydrothermal Synthesis. Crystals, 11(10), 1198. https://doi.org/10.3390/cryst11101198