Halogen Bonding in N-Alkyl-3-halogenopyridinium Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solution and Single Crystal Synthesis of Cocrystals
2.2. Powder X-ray Diffraction Measurements
2.3. Single Crystal X-ray Diffraction Measurements
2.4. Thermal Analysis
2.5. Calculations
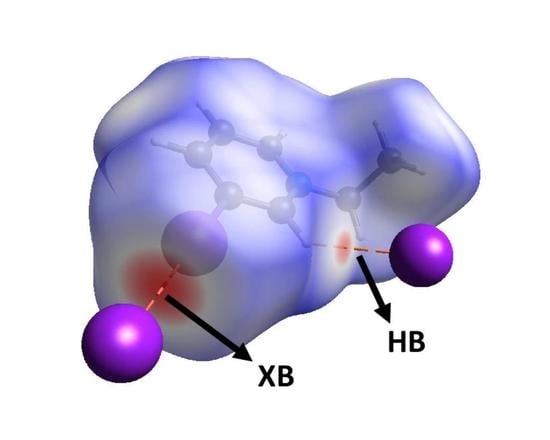
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [Green Version]
- Metrangolo, P.; Resnati, G. Halogen Bonding; Metrangolo, P., Resnati, G., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 126. [Google Scholar]
- Metrangolo, P.; Resnati, G. Halogen bonding: A paradigm in supramolecular chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Erdélyi, M. Halogen bonding in solution. Chem. Soc. Rev. 2012, 41, 3547–3557. [Google Scholar] [CrossRef]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen bonding in supramolecular chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The σ-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef] [PubMed]
- Legon, A.C. The halogen bond: An interim perspective. Phys. Chem. Chem. Phys. 2010, 12, 7736–7747. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Friščić, T.; Jones, W. Structural equivalence of Br and I halogen bonds: A route to isostructural materials with controllable properties. Chem. Mater. 2008, 20, 6623–6626. [Google Scholar] [CrossRef]
- Ding, X.; Tuikka, M.; Haukk, M. Halogen Bonding in Crystal Engineering. In Recent Advances in Crystallography; InTech: London, UK, 2012; Volume 262, pp. 143–168. [Google Scholar]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J. Practical crystal engineering using halogen bonding: A hierarchy based on calculated molecular electrostatic potential surfaces. J. Mol. Struct. 2014, 1072, 20–27. [Google Scholar] [CrossRef]
- Bulfield, D.; Huber, S.M. Halogen Bonding in Organic Synthesis and Organocatalysis. Chem. Eur. J. 2016, 22, 14434–14450. [Google Scholar] [CrossRef]
- Lisac, K.; Topić, F.; Arhangelskis, M.; Cepić, S.; Julien, P.A.; Nickels, C.W.; Morris, A.J.; Friščić, T.; Cinčić, D. Halogen-bonded cocrystallization with phosphorus, arsenic and antimony acceptors. Nat. Commun. 2019, 10, 61. [Google Scholar] [CrossRef]
- Nemec, V.; Lisac, K.; Bedeković, N.; Fotović, L.; Stilinović, V.; Cinčić, D. Crystal engineering strategies towards halogen-bonded metal-organic multi-component solids: Salts, cocrystals and salt cocrystals. CrystEngComm 2021, 23, 3063–3083. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Baldrighi, M.; Desper, J.; Metrangolo, P.; Resnati, G. Supramolecular hierarchy among halogen-bond donors. Chem. Eur. J. 2013, 19, 16240–16247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aakeröy, C.B.; Wijethunga, T.K.; Haj, M.A.; Desper, J.; Moore, C. The structural landscape of heteroaryl-2-imidazoles: Competing halogen-and hydrogen-bond interactions. CrystEngComm 2014, 16, 7218. [Google Scholar] [CrossRef]
- Eraković, M.; Cinčić, D.; Molčanov, K.; Stilinović, V. A Crystallographic Charge Density Study of the Partial Covalent Nature of Strong N⋅⋅⋅Br Halogen Bonds. Angew. Chem. Int. Ed. 2019, 58, 15702–15706. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J.; Đaković, M. Electrostatic Potential Differences and Halogen-Bond Selectivity. Cryst. Growth Des. 2016, 16, 2662–2670. [Google Scholar] [CrossRef]
- Turunen, L.; Warzok, U.; Puttreddy, R.; Beyeh, N.K.; Schalley, C.A.; Rissanen, K. [N⋅⋅⋅I+⋅⋅⋅N] Halogen-Bonded Dimeric Capsules from Tetrakis(3-pyridyl)ethylene Cavitands. Angew. Chem. Int. Ed. 2016, 55, 14033–14036. [Google Scholar] [CrossRef] [PubMed]
- Turunen, L.; Pan, F.; Beyeh, N.K.; Trant, J.F.; Ras, R.H.A.; Rissanen, K. Bamboo-like Chained Cavities and Other Halogen-Bonded Complexes from Tetrahaloethynyl Cavitands with Simple Ditopic Halogen Bond Acceptors. Cryst. Growth Des. 2018, 18, 513–520. [Google Scholar] [CrossRef]
- Saccone, M.; Siiskonen, A.; Fernandez-Palacio, F.; Priimagi, A.; Terraneo, G.; Resnati, G.; Metrangolo, P. Halogen bonding stabilizes a cis-azobenzene derivative in the solid state: A crystallographic study. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 227–233. [Google Scholar] [CrossRef]
- Meazza, L.; Foster, J.A.; Fucke, K.; Metrangolo, P.; Resnati, G.; Steed, J.W. Halogen-bonding-triggered supramolecular gel formation. Nat. Chem. 2013, 5, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Saccone, M.; Cavallo, G.; Metrangolo, P.; Pace, A.; Pibiri, I.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bond directionality translates tecton geometry into self-assembled architecture geometry. CrystEngComm 2013, 15, 3102–3105. [Google Scholar] [CrossRef] [Green Version]
- Jakupec, N.; Fotović, L.; Stilinović, V. The effect of halogen bonding on protonated hexacyanoferrate networks in hexacyanoferrates of halogenopyridines. CrystEngComm 2020, 22, 8142–8150. [Google Scholar] [CrossRef]
- Uran, E.; Fotović, L.; Bedeković, N.; Stilinović, V.; Cinčić, D. The amine group as halogen bond acceptor in cocrystals of aromatic diamines and perfluorinated iodobenzenes. Crystals 2021, 11, 529. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Benton, J.; Desper, J. Stabilizing volatile liquid chemicals using co-crystallization. Chem. Commun. 2015, 51, 2425–2428. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Desper, J. Constructing molecular polygons using halogen bonding and bifurcated N-oxides. CrystEngComm 2014, 16, 28–31. [Google Scholar] [CrossRef] [Green Version]
- Carletta, A.; Zbačnik, M.; Vitković, M.; Tumanov, N.; Stilinović, V.; Wouters, J.; Cinčić, D. Halogen-bonded cocrystals of N-salicylidene Schiff bases and iodoperfluorinated benzenes: Hydroxyl oxygen as a halogen bond acceptor. CrystEngComm 2018, 20, 5332–5339. [Google Scholar] [CrossRef]
- Topić, F.; Lisac, K.; Arhangelskis, M.; Rissanen, K.; Cinčić, D.; Friščić, T. Cocrystal trimorphism as a consequence of the orthogonality of halogen- and hydrogen-bonds synthons. Chem. Commun. 2019, 55, 14066–14069. [Google Scholar] [CrossRef]
- Martinez, V.; Bedeković, N.; Stilinović, V.; Cinčić, D. Tautomeric Equilibrium of an asymmetric β-diketone in halogen-bonded cocrystals with perfluorinated iodobenzenes. Crystals 2021, 11, 699. [Google Scholar] [CrossRef]
- Stilinović, V.; Grgurić, T.; Piteša, T.; Nemec, V.; Cinčić, D. Bifurcated and monocentric halogen bonds in cocrystals of metal(ii) acetylacetonates with p-dihalotetrafluorobenzenes. Cryst. Growth Des. 2019, 19, 1245–1256. [Google Scholar] [CrossRef]
- Nemec, V.; Piteša, T.; Friščić, T.; Cinčić, D. The morpholinyl oxygen atom as an acceptor site for halogen-bonded cocrystallization of organic and metal–organic units. Cryst. Growth Des. 2020, 20, 3617–3624. [Google Scholar] [CrossRef]
- Syssa-Magalé, J.-L.; Boubekeur, K.; Schöllhorn, B. First molecular self-assembly of 1,4-diiodo-tetrafluoro-benzene and a ketone via (O⋯I) non-covalent halogen bonds. J. Mol. Struct. 2005, 737, 103–107. [Google Scholar] [CrossRef]
- Goodwin, M.J.; Steed, B.W.; Yufit, D.S.; Musa, O.M.; Berry, D.J.; Steed, J.W. Halogen and Hydrogen Bonding in Povidone-Iodine and Related Co-Phases. Cryst. Growth Des. 2017, 17, 5552–5558. [Google Scholar] [CrossRef] [Green Version]
- Tothadi, S.; Sanphui, P.; Desiraju, G.R. Obtaining synthon modularity in ternary cocrystals with hydrogen bonds and halogen bonds. Cryst. Growth Des. 2014, 14, 5293–5302. [Google Scholar] [CrossRef]
- Saha, B.K.; Nangia, A.; Jaskólski, M. Crystal engineering with hydrogen bonds and halogen bonds. CrystEngComm 2005, 7, 355. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T. Synthesis of an extended halogen-bonded metal–organic structure in a one-pot mechanochemical reaction that combines covalent bonding, coordination chemistry and supramolecular synthesis. CrystEngComm 2014, 16, 10169–10172. [Google Scholar] [CrossRef]
- Nemec, V.; Fotović, L.; Vitasović, T.; Cinčić, D. Halogen bonding of the aldehyde oxygen atom in cocrystals of aromatic aldehydes and 1,4-diiodotetrafluorobenzene. CrystEngComm 2019, 21, 3251–3255. [Google Scholar] [CrossRef]
- Nemec, V.; Fotović, L.; Friščić, T.; Cinčić, D. A large family of halogen-bonded cocrystals involving metal-organic building blocks with open coordination sites. Cryst. Growth Des. 2017, 17, 6169–6173. [Google Scholar] [CrossRef]
- Zbačnik, M.; Pajski, M.; Stilinović, V.; Vitković, M.; Cinčić, D. The halogen bonding proclivity of the: Ortho-methoxy-hydroxy group in cocrystals of o-vanillin imines and diiodotetrafluorobenzenes. CrystEngComm 2017, 19, 5576–5582. [Google Scholar] [CrossRef]
- Lisac, K.; Cinčić, D. The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding. Crystals 2017, 7, 363. [Google Scholar] [CrossRef] [Green Version]
- Lisac, K.; Cinčić, D. Simple design for metal-based halogen-bonded cocrystals utilizing the M–Cl⋯I motif. CrystEngComm 2018, 20, 5955–5963. [Google Scholar] [CrossRef]
- Zordan, F.; Brammer, L. Water molecules insert into N–H⋯Cl–M hydrogen bonds while M–Cl⋯X–C halogen bonds remain intact in dihydrates of halopyridinium hexachloroplatinates. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Zordan, F.; Purver, S.L.; Adams, H.; Brammer, L. Halometallate and halide ions: Nucleophiles in competition for hydrogen bond and halogen bond formation in halopyridinium salts of mixed halide-halometallate anions. CrystEngComm 2005, 7, 350–354. [Google Scholar] [CrossRef]
- Zordan, F.; Brammer, L.; Sherwood, P. Supramolecular chemistry of halogens: Complementary features of inorganic (M-X) and organic (C-X′) halogens applied to M-X⋯X′-C halogen bond formation. J. Am. Chem. Soc. 2005, 127, 5979–5989. [Google Scholar] [CrossRef] [PubMed]
- Decato, D.A.; Riel, A.M.S.; May, J.H.; Bryantsev, V.S.; Berryman, O.B. Theoretical, solid-state, and solution quantification of the hydrogen bond-enhanced halogen bond. Angew. Chem. 2021, 133, 3729–3736. [Google Scholar] [CrossRef]
- Logothetis, T.A.; Meyer, F.; Metrangolo, P.; Pilati, T.; Resnati, G. Crystal engineering of brominated tectons: N-methyl-3,5-dibromopyridinium iodide gives particularly short C-Br⋯I halogen bonding. New J. Chem. 2004, 28, 760–763. [Google Scholar] [CrossRef]
- Fotović, L.; Stilinović, V. Halogenide anions as halogen and hydrogen bond acceptors in iodopyridinium halogenides. CrystEngComm 2020, 22, 4039–4046. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willett, R.D.; Peterson, K.A.; Twamley, B. The nature of halogen⋯halide synthons: Theoretical and crystallographic studies. J. Phys. Chem. A. 2007, 111, 2319–2328. [Google Scholar] [CrossRef]
- Freytag, M.; Jones, P.G.; Ahrens, B.; Fischer, A.K. Hydrogen bonding and halogen-halogen interactions in 4-halopyridinium halides. New J. Chem. 1999, 23, 1137–1139. [Google Scholar] [CrossRef]
- Willett, R.D.; Awwadi, F.; Butcher, R.; Haddad, S.; Twamley, B. The aryl bromine-halide ion synthon and its role in the control of the crystal structures of tetrahalocuprate (II) ions. Cryst. Growth Des. 2003, 3, 301–311. [Google Scholar] [CrossRef]
- Brammer, L.; Mínguez Espallargas, G.; Adams, H. Involving metals in halogen-halogen interactions: Second-sphere Lewis acid ligands for perhalometallate ions (M-X⋯X’-C). CrystEngComm 2003, 5, 343–345. [Google Scholar] [CrossRef]
- Mínguez Espallargas, G.; Brammer, L.; Sherwood, P. Designing intermolecular interactions between halogenated peripheries of inorganic and organic molecules: Electrostatically directed M-X⋯X′- C halogen bonds. Angew. Chem. Int. Ed. 2006, 45, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Roper, L.C.; Präsang, C.; Kozhevnikov, V.N.; Whitwood, A.C.; Karadakov, P.B.; Bruce, D.W. Experimental and theoretical study of halogen-bonded complexes of DMAP with di-and triiodofluorobenzenes. A complex with a very short N⋯I halogen bond. Cryst. Growth Des. 2010, 10, 3710–3720. [Google Scholar] [CrossRef]
- Ding, X.H.; Chang, Y.Z.; Ou, C.J.; Lin, J.Y.; Xie, L.H.; Huang, W. Halogen bonding in the co-crystallization of potentially ditopic diiodotetrafluorobenzene: A powerful tool for constructing multicomponent supramolecular assemblies. Natl. Sci. Rev. 2020, 7, 1906–1932. [Google Scholar] [CrossRef]
- Lunghi, A.; Cardillo, P.; Messina, T.; Metrangolo, P.; Panzeri, W.; Resnati, G. Perfluorocarbon-hydrocarbon self assembling. Thermal and vibrational analyses of one-dimensional networks formed by α,ω-diiodoperfluoroalkanes with K.2.2. and K.2.2.2. J. Fluor. Chem. 1998, 91, 191–194. [Google Scholar] [CrossRef]
- Messina, M.T.; Metrangolo, P.; Panzeri, W.; Ragg, E.; Resnati, G. Perfluorocarbon-hydrocarbon self-assembly. Part 3. Liquid phase interactions between perfluoroalkylhalides and heteroactom containing hydrocarbons. Tetrahedron Lett. 1998, 39, 9069–9072. [Google Scholar] [CrossRef]
- Farina, A.; Meille, S.V.; Messina, M.T.; Metrangolo, P.; Resnati, G.; Vecchio, G. Resolution of racemic 1,2-dibromohexafluoropropane through halogen- bonded supramolecular helices. Angew. Chem. Int. Ed. 1999, 38, 2433–2436. [Google Scholar] [CrossRef]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. —Int. Ed. 2008, 47, 6114–6127. [Google Scholar] [CrossRef]
- Raatikainen, K.; Rissanen, K. Hierarchical halogen bonding induces polymorphism. CrystEngComm 2009, 11, 750–752. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Ellington, T.L.; Allen, K.E.; Gorden, J.D.; Rheingold, A.L.; Tschumper, G.S.; Hammer, N.I.; Watkins, D.L. Systematic experimental and computational studies of substitution and hybridization effects in solid-state halogen bonded assemblies. Cryst. Growth Des. 2018, 18, 3244–3254. [Google Scholar] [CrossRef]
- Rosokha, S.V.; Loboda, E.A. Interplay of halogen and π-π charge-transfer bondings in intermolecular associates of bromo- or iododinitrobenzene with tetramethyl-p-phenylenediamine. J. Phys. Chem. A. 2015, 119, 3833–3842. [Google Scholar] [CrossRef]
- Nwachukwu, C.I.; Kehoe, Z.R.; Bowling, N.P.; Speetzen, E.D.; Bosch, E. Cooperative halogen bonding and polarized π-stacking in the formation of coloured charge-transfer co-crystals. New J. Chem. 2018, 42, 10615–10622. [Google Scholar] [CrossRef]
- Baykov, S.V.; Filimonov, S.I.; Rozhkov, A.V.; Novikov, A.S.; Ananyev, I.V.; Ivanov, D.M.; Kukushkin, V.Y. Reverse Sandwich structures from interplay between lone pair-π-hole atom-directed C···dz2[M] and halogen bond interactions. Cryst. Growth Des. 2020, 20, 995–1008. [Google Scholar] [CrossRef]
- Barry, D.E.; Hawes, C.S.; Blasco, S.; Gunnlaugsson, T. Structure Direction, solvent effects, and anion influences in halogen-bonded adducts of 2,6-bis(iodoethynyl)pyridine. Cryst. Growth Des. 2016, 16, 5194–5205. [Google Scholar] [CrossRef]
- Nguyen, S.T.; Rheingold, A.L.; Tschumper, G.S.; Watkins, D.L. Elucidating the effects of fluoro and nitro substituents on halogen bond driven assemblies of pyridyl-capped π-conjugated molecules. Cryst. Growth Des. 2016, 16, 6648–6653. [Google Scholar] [CrossRef]
- Baldrighi, M.; Bartesaghi, D.; Cavallo, G.; Chierotti, M.R.; Gobetto, R.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Polymorphs and co-crystals of haloprogin: An antifungal agent. CrystEngComm 2014, 16, 5897–5904. [Google Scholar] [CrossRef] [Green Version]
- Stilinović, V.; Horvat, G.; Hrenar, T.; Nemec, V.; Cinčić, D. Halogen and hydrogen bonding between (N-halogeno)-succinimides and pyridine derivatives in solution, the solid state and in silico. Chem. Eur. J. 2017, 23, 5244–5257. [Google Scholar] [CrossRef]
- Raatikainen, K.; Rissanen, K. Interaction between amines and N-haloimides: A new motif for unprecedentedly short Br⋯N and I⋯N halogen bonds. CrystEngComm 2011, 13, 6972–6977. [Google Scholar] [CrossRef]
- Raatikainen, K.; Rissanen, K. Breathing molecular crystals: Halogen-and hydrogen-bonded porous molecular crystals with solvent induced adaptation of the nanosized channels. Chem. Sci. 2012, 3, 1235–1239. [Google Scholar] [CrossRef]
- Makhotkina, O.; Lieffrig, J.; Jeannin, O.; Fourmigué, M.; Aubert, E.; Espinosa, E. Cocrystal or salt: Solid state-controlled iodine shift in crystalline halogen-bonded systems. Cryst. Growth Des. 2015, 15, 3464–3473. [Google Scholar] [CrossRef]
- Puttreddy, R.; Jurček, O.; Bhowmik, S.; Mäkelä, T.; Rissanen, K. Very strong −N–X+⋯−O–N+ halogen bonds. Chem. Commun. 2016, 52, 2338–2341. [Google Scholar] [CrossRef]
- Puttreddy, R.; Rautiainen, J.M.; Mäkelä, T.; Rissanen, K. Strong N−X⋅⋅⋅O−N halogen bonds: A comprehensive study on N-halosaccharin pyridine N-oxide complexes. Angew. Chem. Int. Ed. 2019, 58, 18610–18618. [Google Scholar] [CrossRef]
- Mavračić, J.; Cinčić, D.; Kaitner, B. Halogen bonding of: N-bromosuccinimide by grinding. CrystEngComm 2016, 18, 3343–3346. [Google Scholar] [CrossRef] [Green Version]
- Dolenc, D.; Modec, B. EDA Complexes of N-halosaccharins with N- and O-donor ligands. New J. Chem. 2009, 33, 2344–2349. [Google Scholar] [CrossRef]
- Yu, S.; Ward, J.S.; Truong, K.; Rissanen, K. Carbonyl hypoiodites as extremely strong halogen bond donors. Angew. Chem. Int. Ed. 2021, 60, 20739–20743. [Google Scholar] [CrossRef]
- Aubert, E.; Espinosa, E.; Nicolas, I.; Jeannin, O.; Fourmigué, M. Toward a reverse hierarchy of halogen bonding between bromine and iodine. Faraday Discuss. 2017, 203, 389–406. [Google Scholar] [CrossRef] [Green Version]
- Thomas, L.H.; Adam, M.S.; O’Neill, A.; Wilson, C.C. Utilizing proton transfer to produce molecular salts in bromanilic acid substituted-pyridine molecular complexes-predictable synthons? Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 1279–1288. [Google Scholar] [CrossRef]
- Espallargas, G.M.; Zordan, F.; Marín, L.A.; Adams, H.; Shankland, K.; van de Streek, J.; Brammer, L. Rational modification of the hierarchy of intermolecular interactions in molecular crystal structures by using tunable halogen Bonds. Chem. Eur. J. 2009, 15, 7554–7568. [Google Scholar] [CrossRef] [PubMed]
- Awwadi, F.F.; Willett, R.D.; Twamley, B. The aryl chlorine-halide ion synthon and its role in the control of the crystal structures of tetrahalocuprate(II) ions. Cryst. Growth Des. 2007, 7, 624–632. [Google Scholar] [CrossRef]
- Vitorica-Yrezabal, I.J.; Sullivan, R.A.; Purver, S.L.; Curfs, C.; Tang, C.C.; Brammer, L. Synthesis and polymorphism of (4-ClpyH)2[CuCl4]: Solid-gas and solid-solid reactions. CrystEngComm 2011, 13, 3189–3196. [Google Scholar] [CrossRef]
- Wolf, J.; Huber, F.; Erochok, N.; Heinen, F.; Guérin, V.; Legault, C.Y.; Kirsch, S.F.; Huber, S.M. Activation of a metal-halogen bond by halogen bonding. Angew. Chem. Int. Ed. 2020, 59, 16496–16500. [Google Scholar] [CrossRef]
- Sutar, R.L.; Engelage, E.; Stoll, R.; Huber, S.M. Bidentate chiral bis(imidazolium)-based halogen-bond donors: Synthesis and applications in enantioselective recognition and catalysis. Angew. Chem. Int. Ed. 2020, 59, 6806–6810. [Google Scholar] [CrossRef] [Green Version]
- Derossi, S.; Brammer, L.; Hunter, C.A.; Ward, M.D. Halogen bonded supramolecular assemblies of [Ru(bipy)(CN)4]2− anions and N-methyl-halopyridinium cations in the solid state and in solution. Inorg. Chem. 2009, 48, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Ormond-Prout, J.E.; Smart, P.; Brammer, L. Cyanometallates as halogen bond acceptors. Cryst. Growth Des. 2012, 12, 205–216. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Willett, R.D.; Twamley, B. The Role of Charge Assisted Arylhalogen-Halide Ion Interactions in the Structures of the Dibromopyridinium Halide Salts. J. Mol. Struct. 2009, 918, 116–122. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Taher, D.; Kailani, M.H.; Alwahsh, M.I.; Odeh, F.; Rüffer, T.; Schaarschmidt, D.; Lang, H. Halogen Bonding Interactions in Halopyridine–Iodine Monochloride Complexes. Cryst. Growth Des. 2020, 20, 543–551. [Google Scholar] [CrossRef]
- Awwadi, F.F.; Taher, D.; Maabreh, A.; Alwedian, F.Z.; Al-Ebaisat, H.; Rüffer, T.; Lang, H. The Role of Fe–X···X–Fe Contacts in the Crystal Structures of [(2-Iodopyridinium)2FeX4]X (X = Cl, Br). Struct. Chem. 2013, 24, 401–408. [Google Scholar] [CrossRef]
- Bondarenko, M.A.; Adonin, S.A.; Novikov, A.S.; Sokolov, M.N.; Fedin, V.P. Supramolecular Bromoantimonate(V) Polybromide (2,6-BrPyH)3[SbBr6]{(Br2)Br} · 2H2O: Specific Features of Halogen···Halogen Contacts in the Crystal Structure. Russ. J. Coord. Chem. 2020, 46, 302–307. [Google Scholar] [CrossRef]
- Anagnostis, J.; Cipi, J.; Landee, C.P.; Tremelling, G.W.; Turnbull, M.M.; Twamley, B.; Wikaira, J.L. Transition Metal Salts of 2-Amino-3,5-Dihalopyridine—Dimers: Syntheses, Structures and Magnetic Properties of (3,5-DiCAPH)2Cu2Br6 and (3,5-DiBAPH)2Cu2 × 6. J. Coord. Chem. 2017, 70, 3892–3906. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fusco, N.; La Rocca, M.V.; Linati, L.; Lo Presti, E.; Mella, M.; Metrangolo, P.; Miljkovic, A. Novel hydrogen- and halogen-bonding anion receptors based on 3-iodopyridinium units. RSC Adv. 2016, 6, 67540–67549. [Google Scholar] [CrossRef] [Green Version]
- Riel, A.M.S.; Jessop, M.J.; Decato, D.A.; Massena, C.J.; Nascimento, V.R.; Berryman, O.B. Experimental investigation of halogen-bond hard-soft acid-base complementarity. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Riel, A.M.S.; Decato, D.A.; Sun, J.; Massena, C.J.; Jessop, M.J.; Berryman, O.B. The intramolecular hydrogen bonded-halogen bond: A new strategy for preorganization and enhanced binding. Chem. Sci. 2018, 9, 5828–5836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foyle, É.M.; White, N.G. Anion templated crystal engineering of halogen bonding tripodal tris(halopyridinium) compounds. CrystEngComm 2020, 22, 2526–2536. [Google Scholar] [CrossRef]
- Lohrman, J.A.; Deng, C.L.; Shear, T.A.; Zakharov, L.N.; Haley, M.M.; Johnson, D.W. Methanesulfonyl-polarized halogen bonding enables strong halide recognition in an arylethynyl anion receptor. Chem. Commun. 2019, 55, 1919–1922. [Google Scholar] [CrossRef]
- Jungbauer, S.H.; Huber, S.M. Cationic multidentate halogen-bond donors in halide abstraction organocatalysis: Catalyst optimization by preorganization. J. Am. Chem. Soc. 2015, 137, 12110–12120. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Samsonenko, D.G.; Yushina, I.V.; Sokolov, M.N.; Fedin, V.P. Halobismuthates with Halopyridinium Cations: Appearance or Non-Appearance of Unusual Colouring. CrystEngComm 2018, 20, 7766–7772. [Google Scholar] [CrossRef]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Fedin, V.P. Halogen Contacts-Induced Unusual Coloring in Bi III Bromide Complex: Anion-to-Cation Charge Transfer via Br⋅⋅⋅Br Interactions. Chem. A Eur. J. 2017, 23, 15612–15616. [Google Scholar] [CrossRef]
- Kosaka, Y.; Yamamoto, H.M.; Nakao, A.; Tamura, M.; Kato, R. Coexistence of conducting and magnetic electrons based on molecular π-electrons in the supramolecular conductor (Me-3,5-DIP)[Ni(dmit)2]2. J. Am. Chem. Soc. 2007, 129, 3054–3055. [Google Scholar] [CrossRef]
- Kusamoto, T.; Yamamoto, H.M.; Tajima, N.; Oshima, Y.; Yamashita, S.; Kato, R. Bilayer mott system based on Ni(dmit)2 (dmit = 1,3-dithiole-2-thione-4,5-dithiolate) anion radicals: Two isostructural salts exhibit contrasting magnetic behavior. Inorg. Chem. 2012, 51, 11645–11654. [Google Scholar] [CrossRef]
- Kosaka, Y.; Yamamoto, H.M.; Tajima, A.; Nakao, A.; Cui, H.; Kato, R. Supramolecular Ni(dmit)2 salts with halopyridinium cations-development of multifunctional molecular conductors with the use of competing supramolecular interactions. CrystEngComm 2013, 15, 3200–3211. [Google Scholar] [CrossRef]
- Ren, X.; Meng, Q.; Song, Y.; Lu, C.; Hu, C.; Chen, X. Unusual magnetic properties of one-dimensional molecule-based magnets associated with a structural phase transition. Inorg. Chem. 2002, 41, 5686–5692. [Google Scholar] [CrossRef] [PubMed]
- Fotović, L.; Stilinović, V. Evaluation of halogenopyridinium cations as halogen bond donors. Cryst. Growth Des. Unpublished work.
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- CrysAlis PRO CCD. User Inspired Software for Single Crystal X-ray Diffractometers; Rigaku Corporation: Tokyo, Japan, 2014. [Google Scholar]
- Manual, U. CrysAlis Pro. Power. 2010. Available online: https://www.agilent.com/cs/library/usermanuals/Public/CrysAlis_Pro_User_Manual.pdf (accessed on 17 July 2021).
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX suite for small-molecule single-crystal crystallography. J. Appl. Crystallogr. 1999, 32, 837–838. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van De Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- STARe Software, v.15.00. Thermal Analysis Software; Mettler Toledo: Greifensee, Switzerland, 2016. [Google Scholar]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Peverati, R.; Truhlar, D.G. An improved and broadly accurate local approximation to the exchange–correlation density functional: The MN12-L functional for electronic structure calculations in chemistry and physics. Phys. Chem. Chem. Phys. 2012, 14, 13171. [Google Scholar] [CrossRef] [PubMed]
- Yurieva, A.G.; Poleshchuk, O.K.; Filimonov, V.D. Comparative analysis of a full-electron basis set and pseudopotential for the iodine atom in DFT quantum-chemical calculations of iodine-containing compounds. J. Struct. Chem. 2008, 49, 548–552. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Gilli, G.; Gilli, P. Towards an unified hydrogen-bond theory. J. Mol. Struct. 2000, 552, S0022–S2860. [Google Scholar] [CrossRef]
- Parr, R.G.; Chattaraj, P.K. Principle of maximum hardness. J. Am. Chem. Soc. 1991, 113, 1854–1855. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Lee, H.; Parr, R.G. HSAB principle. J. Am. Chem. Soc. 1991, 113, 1855–1856. [Google Scholar] [CrossRef]











| N-ethylated | N-methylated | |||||
|---|---|---|---|---|---|---|
| d(XB)/Å | R.S. (XB)/% | ESP (X)/kJ mol−1 e−1 | d(XB)/Å | R.S. (XB) | ESP (X)/kJ mol−1 e−1 | |
| 3ClPy | / | / | 294 | 3.776 | –1.2 | 381 |
| 3BrPy | 3.611 | 5.7 | 449 | 3.637 | 5.0 | 452 |
| 3IPy | 3.473 | 12.3 | 570 | 3.538 | 10.7 | 554 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fotović, L.; Stilinović, V. Halogen Bonding in N-Alkyl-3-halogenopyridinium Salts. Crystals 2021, 11, 1240. https://doi.org/10.3390/cryst11101240
Fotović L, Stilinović V. Halogen Bonding in N-Alkyl-3-halogenopyridinium Salts. Crystals. 2021; 11(10):1240. https://doi.org/10.3390/cryst11101240
Chicago/Turabian StyleFotović, Luka, and Vladimir Stilinović. 2021. "Halogen Bonding in N-Alkyl-3-halogenopyridinium Salts" Crystals 11, no. 10: 1240. https://doi.org/10.3390/cryst11101240
APA StyleFotović, L., & Stilinović, V. (2021). Halogen Bonding in N-Alkyl-3-halogenopyridinium Salts. Crystals, 11(10), 1240. https://doi.org/10.3390/cryst11101240