Synthesis, Crystal Structures, and Molecular Properties of Three Nitro-Substituted Chalcones
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. X-ray Crystallography
2.3. General Procedure: Synthesis of Nitro Chalcones Derivatives (1a–1c)
3. Results and Discussion
3.1. Chemistry
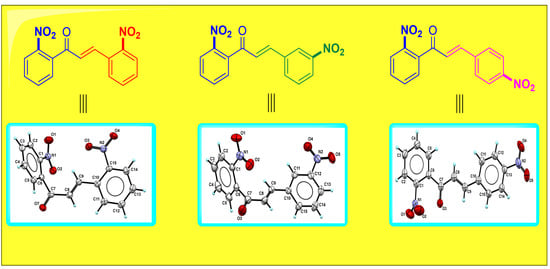
3.2. Structural Description
3.3. Supramolecular Features
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A privileged structure in medicinal chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Rivière, C. Dihydrochalcones: Occurrence in the plant kingdom, chemistry and biological activities. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 51, Chapter 7; pp. 253–381. [Google Scholar]
- Sharma, V.; Singh, G.; Kaur, H.; Saxena, A.K.; Ishar, M.P.S. Synthesis of β-ionone derived chalcones as potent antimicrobial agents. Bioorg. Med. Chem. Lett. 2012, 22, 6343–6346. [Google Scholar] [CrossRef] [PubMed]
- Bondock, S.; Naser, T.; Ammar, Y.-A. Synthesis of some new 2-(3-pyridyl)-4,5-disubstituted thiazoles as potent antimicrobial agents. Eur. J. Med. Chem. 2013, 62, 270–279. [Google Scholar] [CrossRef]
- Damazio, R.G.; Zanatta, A.P.; Cazarolli, L.H.; Mascarello, A.; Chiaradia, L.D.; Nunes, R.J.; Yunes, R.A.; Silva, F.R.M.B. Nitrochalcones: Potential in vivo insulin secretagogues. Biochimie 2009, 91, 1493–1498. [Google Scholar] [CrossRef]
- Tajammal, A.; Batool, M.; Ramzan, A.; Samra, M.M.; Mahnoor, I.; Verpoort, F.; Irfan, A.; Al-Sehemi, A.G.; Munawar, M.A.; Basra, M.A.R. Synthesis, antihyperglycemic activity and computational studies of antioxidant chalcones and flavanones derived from 2, 5 dihydroxyacetophenone. J. Mol. Struct. 2017, 1148, 512–520. [Google Scholar] [CrossRef]
- Higgs, J.; Wasowski, C.; Marcos, A.; Jukič, M.; Paván, C.H.; Gobec, S.; de Tezanos Pinto, F.; Colettis, N.; Marder, M. Chalcone derivatives: Synthesis, in vitro and in vivo evaluation of their anti-anxiety, anti-depression and analgesic effects. Heliyon 2019, 5, e01376. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Duan, D.; Ge, C.; Yao, J.; Liu, Y.; Li, X.; Fang, J. Synthesis of xanthohumol analogues and discovery of potent thioredoxin reductase inhibitor as potential anticancer agent. J. Med. Chem. 2015, 58, 1795–1805. [Google Scholar] [CrossRef]
- Mai, C.W.; Yaeghoobi, M.; Abd-Rahman, N.; Kang, Y.B.; Pichika, M.R. Chalcones with electron-withdrawing and electron-donating substituents: Anticancer activity against TRAIL resistant cancer cells, structure-activity relationship analysis and regulation of apoptotic proteins. Eur. J. Med. Chem. 2014, 77, 378–387. [Google Scholar] [CrossRef]
- Jardim, G.A.M.; Guimarães, T.T.; Pinto, M.C.F.R.; Cavalcanti, B.C.; de Farias, K.M.; Pessoa, C.; Gatto, C.C.; Nair, D.K.; Namboothiri, I.N.N.; da Silva Júnior, E.N. Naphthoquinone-based chalcone hybrids and derivatives: Synthesis and potent activity against cancer cell lines. Med. Chem. Commun. 2015, 6, 120–130. [Google Scholar] [CrossRef]
- Bandeira, P.N.; Lemos, T.L.G.; Santos, H.S.; Carvalho, M.C.S.; Pinheiro, D.P.; Morais-Filho, M.O.; Pessoa, C.; Barros-Nepomuceno, F.W.A.; Rodrigues, T.H.; Ribeiro, P.R.V.; et al. Synthesis, structural characterization, and cytotoxic evaluation of chalcone derivatives. Med. Chem. Res. 2019, 28, 2037. [Google Scholar] [CrossRef]
- Gómez-Rivera, A.; Aguilar-Mariscal, H.; Romero-Ceronio, N.; Roa-de la Fuente, L.F.; Lobato-Garcia, C.E. Synthesis and anti-inflammatory activity of three nitro chalcones. Bioorg. Med. Chem. Lett. 2013, 23, 5519–5522. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Lin, L.; Yang, K.; Wang, S.; Feng, Q.; Zhu, J.; Song, Q. 3-Aminoindole Synthesis from 2-Nitrochalcones and Ammonia or Primary Amines. Adv. Synth. Catal. 2019, 361, 3718–3722. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Retailleau, P. Cooperative Activating Effect of Tertiary Amine-DMSO on Elemental Sulfur: Direct Access to Thioaurones from 2’-Nitrochalcones under Mild Conditions. Org. Lett. 2018, 20, 186–189. [Google Scholar] [CrossRef]
- Poudel, T.N.; Lee, Y.R. Construction of highly functionalized carbazoles via condensation of an enolate to a nitro group. Chem. Sci. 2015, 6, 7028–7033. [Google Scholar] [CrossRef] [Green Version]
- González, J.F.; Rocchi, D.; Tejero, T.; Merino, P.; Menéndez, J.C. One-Pot Synthesis of Functionalized Carbazoles via a CAN-Catalyzed Multicomponent Process Comprising a C—H Activation Step. J. Org. Chem. 2017, 82, 7492–7502. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.B.; Retailleau, P. Redox-Neutral Access to Sultams from 2-Nitrochalcones and Sulfur with Complete Atom Economy. Org. Lett. 2017, 19, 3879–3882. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Retailleau, P. DIPEA-Promoted Reaction of 2-Nitrochalcones with Elemental Sulfur: An Unusual Approach to 2- Benzoylbenzothiophenes. Org. Lett. 2017, 19, 4858–4860. [Google Scholar] [CrossRef]
- Umeda, R.; Kouno, H.; Kitagawa, T.; Okamoto, T.; Kawashima, K.; Mashino, T.; Nishiyama, Y. Selective Synthesis of Quinolines and Indoles: Sulfur-Assisted or Selenium-Catalyzed Reaction of β-(2-Nitrophenyl)-α,β-Unsaturated Ketones with Carbon Monoxide. Heteroat. Chem. 2014, 25, 698–703. [Google Scholar] [CrossRef]
- Lin, Z.; Hu, Z.; Zhang, X.; Dong, J.; Liu, J.-B.; Chen, D.-Z.; Xu, X. Tandem Synthesis of Pyrrolo[2,3-b]quinolones via Cadogen-Type Reaction. Org. Lett. 2017, 19, 5284–5287. [Google Scholar] [CrossRef]
- Aksenov, N.A.; Aksenov, D.A.; Arutiunov, N.A.; Aksenova, D.S.; Aksenov, A.V.; Rubin, M. Unexpected Cyclization of ortho-nitrochalcones into 2-Alkylideneindolin-3-ones. RSC Adv. 2020, 10, 18440–18450. [Google Scholar] [CrossRef]
- Shan, Y.; Liu, Z.; Cao, D.; Sun, Y.; Wang, K.; Chen, H. Nitro substituted chalcone derivatives as quick-response chemosensors for cyanide anions. Sens. Actuators B Chem. 2014, 198, 15–19. [Google Scholar] [CrossRef]
- Simon, F.-X.; Nguyen, T.-T.-T.; Schmutz, M.; Decher, G.; Nicoud, J.-F.; Mésini, P.J. Nitrochalcones as organogelators: Evidence of the involvement of nitro groups and solvent in gel formation. New J. Chem. 2009, 33, 2028–2033. [Google Scholar] [CrossRef]
- Stoe & Cie. X-AREA and X-RED32; Stoe & Cie: Darmstadt, Germany, 2009. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Groom, C.R.; Allen, F.H. The Cambridge Structural Database in retrospect and prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Kinkle, P.; Gibian, H. Uber Chalkone. Chem. Ber. 1961, 94, 26–38. [Google Scholar]
- Prabhu, S.R.; Jayarama, A.; Chandrasekharan, K.; Upadhyaya, V. Synthesis, growth, structural characterization, Hirshfeld analysis and nonlinear optical studies of a methyl substituted chalcone. J. Mol. Struct. 2007, 1136, 244–252. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.H.; Neves, B.J. Chalcone derivatives: Promising starting points for drug design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, I.J.; Cole, J.C.; Kessler, M.; Luo, J.; Sam Motherwell, W.D.; Parkis, L.H.; Smith, B.R.; Taylor, R.; Copper, R.I.; Harris, S.E.; et al. Retrieval of Crystallographic-Derived Molecular Geometry Information. J. Chem. Inf. Comput. Sci. 2004, 44, 2133–2144. [Google Scholar] [CrossRef] [PubMed]
- Jungk, A.E.; Schmidt, G.M.J. Conformational studies. Part II. Crystal and molecular structures of 3-bromo-, 3-chloro-, and 4-bromo-2′-nitrochalcone. J. Chem. Soc. B Phys. Org. 1970, 1427–1434. [Google Scholar] [CrossRef]
- Carpy, A.; Leger, J.M.; Nuhrich, A. 1-(2-nitrophenyl)-3-(5-nitro-2-furanyl)-2-propen-1-one, C13H8N2O6. Cryst. Struct. Commun. 1978, 7, 361–364. [Google Scholar]
- Jezuita, A.; Ejsmont, K.; Szatylowicz, H. Substituent effects of nitro group in cyclic compounds. Struct. Chem. 2021, 32, 179–203. [Google Scholar] [CrossRef]
- Hussein, H.A.; Fadhil, G.F. Theoretical investigation of para amino-dichloro chalcone isomers, part I: A DFT structure—stability study. J. Phys. Org. Chem. 2020, 33, e4073. [Google Scholar] [CrossRef]
- Zainuri, D.A.; Razak, I.A.; Arshad, S. Molecular structure, DFT studies and UV-Vis absorption of two new linear fused ring chalcones:(E)-1-(anthracen-9-yl)-3-(2-methoxyphenyl) prop-2-en-1-one and (E)-1-(anthracen-9-yl)-3-(3-fluoro-4-methoxyphenyl) prop-2-en-1-one. Acta Crystallogr. Sec. E Crystallog. Commun. 2018, 74, 1087–1092. [Google Scholar] [CrossRef]
- Ramos, R.R.; da Silva, C.C.; Guimarães, F.F.; Martins, F.T. Polymorphism and conformerism in chalcones. CrystEngComm 2016, 18, 2144–2154. [Google Scholar] [CrossRef]
- Yu, F.; Wang, M.; Sun, H.; Shan, Y.; Du, M.; Khan, A.; Usman, R.; Zhang, W.; Shan, H.; Xu, C. Tunning the Solid-State Fluoresence of Chalcone Crystals via Molecular Coplanarity and J-Aggregate. RSC Adv. 2017, 7, 8491–8503. [Google Scholar] [CrossRef] [Green Version]
- Almeida, L.R.; Anjos, M.M.; Ribeiro, G.C.; Valverde, C.; Machado, D.F.S.; Oliveira, G.R.; Napolitano, H.B.; de Oliveira, H.C.B. Synthesis, structural characterization and computational study of a novel amino chalcone: A potential nonlinear optical material. New J. Chem. 2017, 41, 1744–1754. [Google Scholar] [CrossRef]
- Kumar, C.S.C.; Loh, W.S.; Ooi, C.W.; Quah, C.K.; Fun, H.K. Heteroaryl chalcones: Design, synthesis, X-ray crystal structures and biological evaluation. Molecules 2013, 18, 12707–12724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakarić, D.; Baranović, G. The conformational equilibrium and vibrational properties of chalcone. J. Mol. Struct. 2019, 1196, 429–438. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; Mckinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A program for Hirshfeld surface analysis, visualization and quantitative analysis of molecular crystal. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer Model Energies and Energy Frameworks: Extension to Metal Coordination Compounds, Organic Salts, Solvates and Open-Shell Systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Tan, S.L.; Jotani, M.M.; Tiekink, E.R. Utilizing Hirshfeld surface calculations, non-covalent interaction (NCI) plots and the calculation of interaction energies in the analysis of molecular packing. Acta Crystallogr. Sec. E Crystallog. Commun. 2019, 75, 308–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, A.; Barr, G.; Dong, W.; Gilmore, C.J.; Jayatilaka, D.; Mckinnon, J.J.; Spackman, M.A.; Wilson, C.C. Comparing entire crystal structure: Structural genetic fingerprint. CrystEngComm 2007, 9, 648–652. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C. Encoding and decoding hydrogen-bond patterns of organic compounds. Acc. Chem. Res. 1990, 23, 120–126. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards Quantitative Analysis of Intermolecular Interactions with Hirshfeld Surfaces. Chem. Commun. 2007, 3814–3816. [Google Scholar] [CrossRef] [PubMed]







| 1a | 1b | 1c | |
|---|---|---|---|
| Empirical formula | C15H10N2O5 | C15H10N2O5 | C15H10N2O5 |
| Formula weight | 298.25 | 298.25 | 298.25 |
| Crystal system | Triclinic | Orthorhombic | Triclinic |
| T (K) | 123(1) | 295(1) | 295(1) |
| Space group | P | Pbca | P |
| CCDC-Numbers | 2036696 | 2036697 | 2036695 |
| Conformation | s-trans | s-trans | s-cis |
| a [Å] | 7.6303(4) | 11.1553(4) | 7.6817(9) |
| b [Å] | 7.8424(5) | 14.1772(5) | 7.8867(7) |
| c [Å] | 12.5262(8) | 17.6747(8) | 12.4081(13) |
| α (deg) | 94.327(5) | 90 | 84.587(8) |
| β (deg) | 90.696(5) | 90 | 74.210(9) |
| γ (deg) | 117.716(4) | 90 | 69.877(8) |
| V (Å3) | 660.72(7) | 2795.27(19) | 679.20(13) |
| Z | 2 | 8 | 2 |
| Radiation type | 0.56083 Å | 0.56083 | 0.56083 |
| θ range | 2.430 to 23.000° | 2.320 to 21.498° | 2.553 to 21.497° |
| Dcalc. (g/cm3) | 1.499 | 1.417 | 1.458 |
| (mm−1) | 0.070 | 0.067 | 0.068 |
| Transm. factors | 0.572–1.000 | 0.428–1.000 | 0.429–1.000 |
| Reflections collected | 16132 | 61499 | 14368 |
| Independent reflections | 3743 | 3270 | 3166 |
| Parameters | 199 | 200 | 209 |
| Rint | 0.0241 | 0.0527 | 0.0429 |
| Goodness-of-fit on F2 | 1.086 | 1.012 | 0.892 |
| Final R index [I > 2σ(I)] | 0.0354 | 0.0386 | 0.0424 |
| wR2 (all data) | 0.1002 | 0.1119 | 0.1199 |
| Largest diff. peak and hole (e/Å3) | 0.341, −0.235 | 0.166, −0.174 | 0.230, −0.187 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidalgo, A.Y.; Velasco, M.; Sánchez-Lara, E.; Gómez-Rivera, A.; Vilchis-Reyes, M.A.; Alvarado, C.; Herrera-Ruiz, M.; López-Rodríguez, R.; Romero-Ceronio, N.; Lobato-García, C.E. Synthesis, Crystal Structures, and Molecular Properties of Three Nitro-Substituted Chalcones. Crystals 2021, 11, 1589. https://doi.org/10.3390/cryst11121589
Hidalgo AY, Velasco M, Sánchez-Lara E, Gómez-Rivera A, Vilchis-Reyes MA, Alvarado C, Herrera-Ruiz M, López-Rodríguez R, Romero-Ceronio N, Lobato-García CE. Synthesis, Crystal Structures, and Molecular Properties of Three Nitro-Substituted Chalcones. Crystals. 2021; 11(12):1589. https://doi.org/10.3390/cryst11121589
Chicago/Turabian StyleHidalgo, Alam Yair, Manuel Velasco, Eduardo Sánchez-Lara, Abraham Gómez-Rivera, Miguel A. Vilchis-Reyes, Cuauhtémoc Alvarado, Maribel Herrera-Ruiz, Ricardo López-Rodríguez, Nancy Romero-Ceronio, and Carlos E. Lobato-García. 2021. "Synthesis, Crystal Structures, and Molecular Properties of Three Nitro-Substituted Chalcones" Crystals 11, no. 12: 1589. https://doi.org/10.3390/cryst11121589
APA StyleHidalgo, A. Y., Velasco, M., Sánchez-Lara, E., Gómez-Rivera, A., Vilchis-Reyes, M. A., Alvarado, C., Herrera-Ruiz, M., López-Rodríguez, R., Romero-Ceronio, N., & Lobato-García, C. E. (2021). Synthesis, Crystal Structures, and Molecular Properties of Three Nitro-Substituted Chalcones. Crystals, 11(12), 1589. https://doi.org/10.3390/cryst11121589