1D Perovskitoid as Absorbing Material for Stable Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Device Fabrication
2.3. Characterizations
3. Results
3.1. Fabrication and Characterizations of 1D Perovskitoid Films
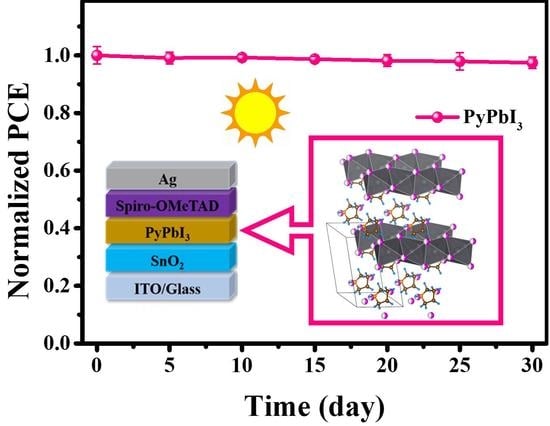
3.2. Stability Measurements of 1D Perovskitoid Films
3.3. Photovoltaic Performance of 1D Perovskitoid Solar Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rong, Y.; Hu, Y.; Mei, A.; Tan, H.; Saidaminov, M.I.; Seok, S.I.; McGehee, M.D.; Sargent, E.H.; Han, H. Challenges for commercializing perovskite solar cells. Science 2018, 361, eaat8235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Wang, L.; Xu, F.; Wu, J.; Song, T.; Chen, Q. Recent Progress in Developing Monolithic Perovskite/Si Tandem Solar Cells. Front. Chem. 2020, 8, 8. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Graẗzel, M.; Mhaisalkar, S.; Sum, T.C. Long-range balanced electronand hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Yin, W.-J.; Yang, J.-H.; Kang, J.; Yan, Y.; Wei, S.-H. Halide perovskite materials for solar cells: A theoretical review. J. Mater. Chem. A 2014, 3, 8926–8942. [Google Scholar] [CrossRef]
- Wang, R.T.; Xu, A.F.; Yang, L.W.; Chen, J.Y.; Kitai, A.; Xu, G. Magnetic-field-induced energy bandgap reduction of perovskite KMnF3. J. Mater. Chem. C 2020, 8, 4164–4168. [Google Scholar] [CrossRef]
- Xu, K.J.; Wang, R.T.; Xu, A.F.; Chen, J.Y.; Xu, G. Hysteresis and Instability Predicted in Moisture Degradation of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 48882–48889. [Google Scholar] [CrossRef]
- Conings, B.; Drijkoningen, J.; Gauquelin, N.; Babayigit, A.; D’Haen, J.; D’Olieslaeger, L.; Ethirajan, A.; Verbeeck, J.; Manca, J.; Mosconi, E.; et al. Intrinsic Thermal Instability of Methylammonium Lead Trihalide Perovskite. Adv. Energy Mater. 2015, 5, 5. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Bai, Y.; Meng, X.; Yang, S. Interface Engineering for Highly Efficient and Stable Planar p-i-n Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 8. [Google Scholar] [CrossRef]
- Grancini, G.; Roldán-Carmona, C.; Zimmermann, I.; Mosconi, E.; Lee, X.; Martineau, D.; Narbey, S.; Oswald, F.; De Angelis, F.; Graetzel, M.; et al. One-Year stable perovskite solar cells by 2D/3D interface engineering. Nat. Commun. 2017, 8, 15684. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved Phase Stability of Formamidinium Lead Triiodide Perovskite by Strain Relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Zhu, C.; Niu, X.; Fu, Y.; Li, N.; Hu, C.; Chen, Y.; He, X.; Na, G.; Liu, P.; Zai, H.; et al. Strain engineering in perovskite solar cells and its impacts on carrier dynamics. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Meyer, S.; Dkhissi, Y.; Weber, K.; Pringle, J.M.; Bach, U.; Spiccia, L.; Cheng, Y.B. Degradation observations of en-capsulated planar CH3NH3PbI3 perovskite solar cells at high temperatures and humidity. J. Mater. Chem. A 2015, 3, 8139–8147. [Google Scholar] [CrossRef]
- Ma, S.; Bai, Y.; Wang, H.; Zai, H.; Wu, J.; Li, L.; Xiang, S.; Liu, N.; Liu, L.; Zhu, C.; et al. 1000 h Operational Lifetime Perovskite Solar Cells by Ambient Melting Encapsulation. Adv. Energy Mater. 2020, 10, 1902472. [Google Scholar] [CrossRef]
- Pering, S.R.; Deng, W.; Troughton, J.R.; Kubiak, P.S.; Ghosh, D.; Niemann, R.G.; Brivio, F.; Jeffrey, F.E.; Walker, A.B.; Islam, M.S.; et al. Azetidinium lead iodide for perovskite solar cells. J. Mater. Chem. A 2017, 5, 20658–20665. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Rubel, O. Aziridinium Lead Iodide: A Stable, Low-Band-Gap Hybrid Halide Perovskite for Photovoltaics. J. Phys. Chem. Lett. 2018, 9, 874–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tombe, S.; Adam, G.; Heilbrunner, H.; Apaydin, D.H.; Ulbricht, C.; Sariciftci, N.S.; Arendse, C.J.; Iwuoha, E.; Scharber, M.C. Optical and electronic properties of mixed halide (X = I, Cl, Br) methylammonium lead perovskite solar cells. J. Mater. Chem. C 2017, 5, 1714–1723. [Google Scholar] [CrossRef] [Green Version]
- Xu, A.F.; Wang, R.T.; Yang, L.W.; Liu, E.E.; Xu, G. An environmentally stable organic–inorganic hybrid perovskite containing py cation with low trapstate density. Crystals 2020, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, X.; Wang, G.; Tong, J.; Pan, D. All-inorganic and lead-free BiI3 thin film solar cells by iodization of BiSI thin films. J. Mater. Chem. C 2020, 8, 14066–14074. [Google Scholar] [CrossRef]
- Pandian, M.G.M.; Khadka, D.B.; Shirai, Y.; Umedov, S.; Yanagida, M.; Subashchandran, S.; Grigorieva, A.; Miyano, K. Ef-fect of solvent vapour annealing on bismuth triiodide film for photovoltaic applications and its optoelectronic properties. J. Mater. Chem. C 2020, 8, 12173–12180. [Google Scholar] [CrossRef]
- Hu, W.; He, X.; Fang, Z.; Lian, W.; Shang, Y.; Li, X.; Zhou, W.; Zhang, M.; Chen, T.; Lu, Y.; et al. Bulk heterojunction gifts bismuth-based lead-free perovskite solar cells with record efficiency. Nano Energy 2020, 68, 104362. [Google Scholar] [CrossRef]
- Khadka, D.B.; Shirai, Y.; Yanagida, M.; Miyano, K. Tailoring the film morphology and interface band offset of caesium bismuth iodide-based Pb-free perovskite solar cells. J. Mater. Chem. C 2019, 7, 8335–8343. [Google Scholar] [CrossRef]
- McCall, K.M.; Stoumpos, C.C.; Kontsevoi, O.Y.; Alexander, G.C.B.; Wessels, B.W.; Kanatzidis, M.G. From 0D Cs3Bi2I9 to 2D Cs3Bi2I6Cl3: Dimensional Expansion Induces a Direct Band Gap but Enhances Electron–Phonon Coupling. Chem. Mater. 2019, 31, 2644–2650. [Google Scholar] [CrossRef]
- Umar, F.; Zhang, J.; Jin, Z.; Muhammad, I.; Yang, X.; Deng, H.; Jahangeer, K.; Hu, Q.; Song, H.; Tang, J. Dimensionality Controlling of Cs3Sb2I9 for Efficient All-Inorganic Planar Thin Film Solar Cells by HCl-Assisted Solution Method. Adv. Opt. Mater. 2019, 7, 7. [Google Scholar] [CrossRef]
- Xu, A.F.; Wang, R.T.; Yang, L.W.; Jarvis, V.; Britten, J.F.; Xu, G.; Yang, W. Pyrrolidinium lead iodide from crystallography: A new perovskite with low bandgap and good water resistance. Chem. Commun. 2019, 55, 3251–3253. [Google Scholar] [CrossRef]
- Gao, L.; Spanopoulos, I.; Ke, W.; Huang, S.; Hadar, I.; Chen, L.; Li, X.; Yang, G.; Kanatzidis, M.G. Improved Environmental Stability and Solar Cell Efficiency of (MA,FA)PbI3 Perovskite Using a Wide-Band-Gap 1D Thiazolium Lead Iodide Capping Layer Strategy. ACS Energy Lett. 2019, 4, 1763–1769. [Google Scholar] [CrossRef]
- Li, C.; Song, Z.; Chen, C.; Xiao, C.; Subedi, B.; Harvey, S.P.; Shrestha, N.; Subedi, K.K.; Chen, L.; Liu, D.; et al. Low-bandgap mixed tin–lead iodide perovskites with reduced methylammonium for simultaneous enhancement of solar cell efficiency and stability. Nat. Energy 2020, 5, 768–776. [Google Scholar] [CrossRef]
- Xu, A.F.; Wang, R.T.; Yang, L.W.; Liu, N.; Chen, Q.; Lapierre, R.; Isik Goktas, N.; Xu, G. Pyrrolidinium containing perovskites with thermal stability and water resistance for photovoltaics. J. Mater. Chem. C 2019, 7, 11104–11108. [Google Scholar] [CrossRef]
- Xu, A.F.; Liu, N.; Xie, F.; Song, T.; Ma, Y.; Zhang, P.; Bai, Y.; Li, Y.; Chen, Q.; Xu, G. Promoting Thermodynamic and Kinetic Stabilities of FA-based Perovskite by an in Situ Bilayer Structure. Nano Lett. 2020, 20, 3864–3871. [Google Scholar] [CrossRef]
- Pham, N.D.; Yang, Y.; Hoang, M.T.; Wang, T.; Tiong, V.T.; Wilson, G.J.; Wang, H. 1D Pyrrolidinium Lead Iodide for Efficient and Stable Perovskite Solar Cells. Energy Technol. 2020, 8, 1900918. [Google Scholar] [CrossRef]
- Miao, Y.; Fan, H.; Wang, P.; Zhang, Y.; Gao, C.; Yang, L.M.; Song, Y.L.; Yang, C.; Liu, C.M.; Jiang, K. From 1D to 3D: Fabrication of CH3NH3PbI3 Perovskite Solar Cell Thin Films from (Pyrrolidinium)PbI3 via Organic Cation Exchange Approach. Energy Technol. 2020, 8, 2000148. [Google Scholar] [CrossRef]
- Li, N.; Niu, X.; Chen, Q.; Zhou, H. Towards commercialization: The operational stability of perovskite solar cells. Chem. Soc. Rev. 2020, 49, 8235–8286. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, N.; Moon, S.-J.; Humphry-Baker, R.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nat. Cell Biol. 2013, 499, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Ma, Y.; Zhang, C.; Liu, C.; Li, W.; Schropp, R.E.I.; Mai, Y. Thermodynamically Self-Healing 1D–3D Hybrid Perovskite Solar Cells. Adv. Energy Mater. 2018, 8, 1703421. [Google Scholar] [CrossRef]
- Weber, O.J.; Marshall, K.L.; Dyson, L.M.; Weller, M.T. Structural diversity in hybrid organic-inorganic lead iodide materials. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 668–678. [Google Scholar] [CrossRef] [PubMed]
- El-Mellouhi, F.; Marzouk, A.; Bentria, E.T.; Rashkeev, S.N.; Kais, S.; Alharbi, F.H. Hydrogen Bonding and Stability of Hybrid Organic-Inorganic Perovskites. ChemSusChem 2016, 9, 2648–2655. [Google Scholar] [CrossRef]
- Svane, K.L.; Forse, A.C.; Grey, C.P.; Kieslich, G.; Cheetham, A.K.; Walsh, A.; Butler, K.T. How Strong Is the Hydrogen Bond in Hybrid Perovskites? J. Phys. Chem. Lett. 2017, 8, 6154–6159. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Du, Q.; Yin, G.; Liu, P.; Li, L.; Xie, H.; Zhu, C.; Li, Y.; Zhou, H.; Zhang, W.B.; et al. Extremely low trapstate energy level perovskite solar cells passivated using NH2-POSS with improved efficiency and stability. J. Mater. Chem. A 2018, 6, 6806–6814. [Google Scholar] [CrossRef]
- Wang, R.T.; Xu, A.F.; Chen, J.Y.; Yang, L.W.; Xu, G.; Jarvis, V.; Britten, J.F. Reversing Organic-Inorganic Hybrid Perovskite Degradation in Water via pH and Hydrogen Bonds. J. Phys. Chem. Lett. 2019, 10, 7245–7250. [Google Scholar] [CrossRef]
- Eperon, G.E.; Paternò, G.M.; Sutton, R.J.; Zampetti, A.; Haghighirad, A.A.; Cacialli, F.; Snaith, H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A 2015, 3, 19688–19695. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, Y.; Shao, Y.; Yan, Y. Understanding the physical properties of hybrid perovskites for photovoltaic ap-plications. Nat. Rev. Mater. 2017, 2, 1–19. [Google Scholar] [CrossRef]
- Xu, W.; He, F.; Zhang, M.; Nie, P.; Zhang, S.; Zhao, C.; Luo, R.; Li, J.; Zhang, X.; Zhao, S.; et al. Minimizing Voltage Loss in Efficient All-Inorganic CsPbI2Br Perovskite Solar Cells through Energy Level Alignment. ACS Energy Lett. 2019, 4, 2491–2499. [Google Scholar] [CrossRef]
- Matteocci, F.; Cinà, L.; Lamanna, E.; Cacovich, S.; Divitini, G.; Midgley, P.A.; Ducati, C.; Di Carlo, A. Encapsulation for long-term stability enhancement of perovskite solar cells. Nano Energy 2016, 30, 162–172. [Google Scholar] [CrossRef] [Green Version]
- Yi, H.; Wang, D.; Duan, L.; Haque, F.; Xu, C.; Zhang, Y.; Conibeer, G.; Uddin, A. Solution-processed WO3 and water-free PEDOT:PSS composite for hole transport layer in conventional perovskite solar cell. Electrochimica Acta 2019, 319, 349–358. [Google Scholar] [CrossRef]
- Wu, G.; Zhou, C.; Ming, W.; Han, D.; Chen, S.; Yang, D.; Besara, T.; Neu, J.; Siegrist, T.; Du, M.-H.; et al. A One-Dimensional Organic Lead Chloride Hybrid with Excitation-Dependent Broadband Emissions. ACS Energy Lett. 2018, 3, 1443–1449. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, F.; Li, Y.; Liu, N.; Han, Y.; Zou, M.; Song, T. 1D Perovskitoid as Absorbing Material for Stable Solar Cells. Crystals 2021, 11, 241. https://doi.org/10.3390/cryst11030241
Xu F, Li Y, Liu N, Han Y, Zou M, Song T. 1D Perovskitoid as Absorbing Material for Stable Solar Cells. Crystals. 2021; 11(3):241. https://doi.org/10.3390/cryst11030241
Chicago/Turabian StyleXu, Fan, Yujing Li, Na Liu, Ying Han, Meishuai Zou, and Tinglu Song. 2021. "1D Perovskitoid as Absorbing Material for Stable Solar Cells" Crystals 11, no. 3: 241. https://doi.org/10.3390/cryst11030241
APA StyleXu, F., Li, Y., Liu, N., Han, Y., Zou, M., & Song, T. (2021). 1D Perovskitoid as Absorbing Material for Stable Solar Cells. Crystals, 11(3), 241. https://doi.org/10.3390/cryst11030241