Solution Cocrystallization: A Scalable Approach for Cocrystal Production
Abstract
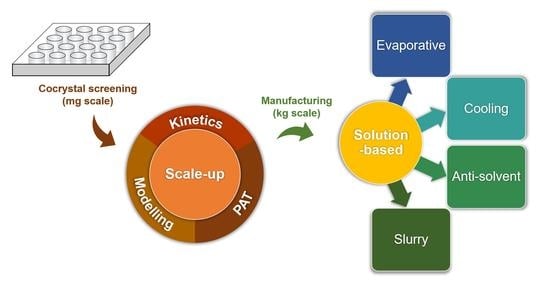
:1. Introduction
2. Types of Solution Cocrystallization
2.1. Evaporative Cocrystallization
2.2. Cooling Cocrystallization
2.3. Antisolvent Cocrystallization
2.4. Slurry Cocrystallization
2.5. Ultrasound-Assisted Cocrystallization
2.6. Supercritical Fluid Cocrystallization
3. Challenges to Industrial Cocrystallization
3.1. Kinetics of Cocrystallization
3.2. Scalable Methods
3.3. Continuous Manufacturing
3.4. Application of Modeling and Process Analytical Technology (PAT)
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Sinha, A.S.; Maguire, A.R.; Lawrence, S.E. Cocrystallization of Nutraceuticals. Cryst. Growth Des. 2015, 15, 984–1009. [Google Scholar] [CrossRef]
- Aakeröy, C. Is There Any Point in Making Co-Crystals? Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2015, 71, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Mazzeo, P.P.; Carraro, C.; Monica, A.; Capucci, D.; Pelagatti, P.; Bianchi, F.; Agazzi, S.; Careri, M.; Raio, A.; Carta, M.; et al. Designing a Palette of Cocrystals Based on Essential Oil Constituents for Agricultural Applications. ACS Sustain. Chem. Eng. 2019, 7, 17929–17940. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Wijethunga, T.K.; Benton, J.; Desper, J. Stabilizing Volatile Liquid Chemicals Using Co-Crystallization. Chem. Commun. 2015, 51, 2425–2428. [Google Scholar] [CrossRef] [PubMed]
- Karagianni, A.; Malamatari, M.; Kachrimanis, K. Pharmaceutical Cocrystals: New Solid Phase Modification Approaches for the Formulation of APIs. Pharmaceutics 2018, 10, 18. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.; Baptista, B.; Lopes, J.A.; Sarraguça, M.C. Pharmaceutical Cocrystallization Techniques. Advances and Challenges. Int. J. Pharm. 2018, 547, 404–420. [Google Scholar] [CrossRef]
- Malamatari, M.; Ross, S.A.; Douroumis, D.; Velaga, S.P. Experimental Cocrystal Screening and Solution Based Scale-up Cocrystallization Methods. Adv. Drug Deliv. Rev. 2017, 117, 162–177. [Google Scholar] [CrossRef]
- Hsi, K.H.-Y.; Chadwick, K.; Fried, A.; Kenny, M.; Myerson, A.S. Separation of Impurities from Solution by Selective Co-Crystal Formation. CrystEngComm 2012, 14, 2386–2388. [Google Scholar] [CrossRef]
- Billot, P.; Hosek, P.; Perrin, M.A. Efficient Purification of an Active Pharmaceutical Ingredient via Cocrystallization: From Thermodynamics to Scale-Up. Org. Process Res. Dev. 2013, 17, 505–511. [Google Scholar] [CrossRef]
- Powell, K.A.; Bartolini, G.; Wittering, K.E.; Saleemi, A.N.; Wilson, C.C.; Rielly, C.D.; Nagy, Z.K. Toward Continuous Crystallization of Urea-Barbituric Acid: A Polymorphic Co-Crystal System. Cryst. Growth Des. 2015, 15, 4821–4836. [Google Scholar] [CrossRef]
- Porter, W.W.; Elie, S.C.; Matzger, A.J. Polymorphism in Carbamazepine Cocrystals. Cryst. Growth Des. 2008, 8, 14–16. [Google Scholar] [CrossRef] [Green Version]
- Leyssens, T.; Tumanova, N.; Robeyns, K.; Candoni, N.; Veesler, S. Solution Cocrystallization, an Effective Tool to Explore the Variety of Cocrystal Systems: Caffeine/Dicarboxylic Acid Cocrystals. CrystEngComm 2014, 16, 9603–9611. [Google Scholar] [CrossRef]
- Kavanagh, O.N.; Croker, D.M.; Walker, G.M.; Zaworotko, M.J. Pharmaceutical Cocrystals: From Serendipity to Design to Application. Drug Discov. Today 2019, 24, 796–804. [Google Scholar] [CrossRef] [Green Version]
- Shan, N.; Zaworotko, M.J. The Role of Cocrystals in Pharmaceutical Science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef]
- Javoor, M.; Mondal, P.; Chopra, D. Cocrystals: A Review of Recent Trends in Pharmaceutical and Material Science Applications. Mater. Sci. Res. India 2017, 14, 09–18. [Google Scholar] [CrossRef]
- Almarsson, Ö.; Peterson, M.L.; Zaworotko, M. The A to Z of Pharmaceutical Cocrystals: A Decade of Fast-Moving New Science and Patents. Pharm. Pat. Anal. 2012, 1, 313–327. [Google Scholar] [CrossRef]
- Bolla, G.; Nangia, A. Pharmaceutical Cocrystals: Walking the Talk. Chem. Commun. 2016, 52, 8342–8360. [Google Scholar] [CrossRef]
- Steed, J.W. The Role of Co-Crystals in Pharmaceutical Design. Trends Pharmacol. Sci. 2013, 34, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Du, S.; Zhang, R.; Jia, X.; Yang, T.; Zhang, X. Drug-Drug Cocrystals: Opportunities and Challenges. Asian J. Pharm. Sci. 2020. [CrossRef]
- Sekhon, B.S. Drug-Drug Co-Crystals. DARU J. Pharm. Sci. 2012, 20, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Grobelny, P.; Mukherjee, A.; Desiraju, G. Drug-Drug Co-Crystals: Temperature-Dependent Proton Mobility in the Molecular Complex of Isoniazid with 4-Aminosalicylic Acid. CrystEngComm 2011, 13, 4304–4306. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Maini, L.; Capucci, D.; Nanna, S.; Wouters, J.; Aerts, L.; Quéré, L. Combining Piracetam and Lithium Salts: Ionic Co-Crystals and Co-Drugs? Chem. Commun. 2012, 48, 8219–8221. [Google Scholar] [CrossRef]
- Bianchi, F.; Fornari, F.; Riboni, N.; Spadini, C.; Cabassi, C.S.; Iannarelli, M.; Carraro, C.; Mazzeo, P.P.; Bacchi, A.; Orlandini, S.; et al. Development of Novel Cocrystal-Based Active Food Packaging by a Quality by Design Approach. Food Chem. 2021, 347, 1–9. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, S.; Gou, R.; Wu, C.; Han, G.; Jia, H. Understanding the Effect of Solvent on the Growth and Crystal Morphology of MTNP/CL-20 Cocrystal Explosive: Experimental and Theoretical Studies. Cryst. Res. Technol. 2018, 53, 1700299. [Google Scholar] [CrossRef]
- Holaň, J.; Štěpánek, F.; Billot, P.; Ridvan, L. The Construction, Prediction and Measurement of Co-Crystal Ternary Phase Diagrams as a Tool for Solvent Selection. Eur. J. Pharm. Sci. 2014, 63, 124–131. [Google Scholar] [CrossRef]
- Leyssens, T.; Springuel, G.; Montis, R.; Candoni, N.; Veesler, S. Importance of Solvent Selection for Stoichiometrically Diverse Cocrystal Systems: Caffeine/Maleic Acid 1:1 and 2:1 Cocrystals. Cryst. Growth Des. 2012, 12, 1520–1530. [Google Scholar] [CrossRef]
- Yu, Q.; Jia, W.; Pu, J.; Wang, Y.; Yang, H. Cocrystallization of Urea and Succinic Acid in “Nano-Crystallizer”. Chem. Eng. Sci. 2021, 229, 116082. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, L.; Yang, W.; Li, Y.; Yang, Y.; Zhang, Z.; Wang, C.; Zhang, X.; Yin, Q. Preparation of Theophylline-Benzoic Acid Cocrystal and On-Line Monitoring of Cocrystallization Process in Solution by Raman Spectroscopy. Crystals 2019, 9, 329. [Google Scholar] [CrossRef] [Green Version]
- Thakor, P.; Yadav, B.; Modani, S.; Shastri, N.R. Preparation and Optimization of Nano-Sized Cocrystals Using a Quality by Design Approach. CrystEngComm 2020, 22, 2304–2314. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, H.; Zhang, J.; Ma, Y.; Tan, Y.; Nie, F.; Zhang, J.; Li, H. Rapid Cocrystallization by Exploiting Differential Solubility: An Efficient and Scalable Process toward Easily Fabricating Energetic Cocrystals. Cryst. Growth Des. 2020, 20, 2129–2134. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, Q.; Zhu, B.; Zhang, Z.; Bao, J.; Ding, Q.; Ren, G.; Mei, X. Pharmaceutical Cocrystals of Nicorandil with Enhanced Chemical Stability and Sustained Release. Cryst. Growth Des. 2020, 20, 6995–7005. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Ren, J.; Zeng, A.; Liu, J. Preparation of Quercetin–Nicotinamide Cocrystals and Their Evaluation under in Vivo and in Vitro Conditions. RSC Adv. 2020, 10, 21852–21859. [Google Scholar] [CrossRef]
- Luo, C.; Liang, W.; Chen, X.; Wang, J.; Deng, Z.; Zhang, H. Pharmaceutical Cocrystals of Naringenin with Improved Dissolution Performance. CrystEngComm 2018, 20, 3025–3033. [Google Scholar] [CrossRef]
- Inam, M.; Wu, J.; Shen, J.; Phan, C.; Tang, G.; Hu, X. Preparation and Characterization of Novel Pharmaceutical Co-Crystals: Ticagrelor with Nicotinamide. Crystals 2018, 8, 336. [Google Scholar] [CrossRef] [Green Version]
- Cuadra, I.A.; Cabañas, A.; Cheda, J.A.R.; Pando, C. Polymorphism in the Co-Crystallization of the Anticonvulsant Drug Carbamazepine and Saccharin Using Supercritical CO2 as an Anti-Solvent. J. Supercrit. Fluids 2018, 136, 60–69. [Google Scholar] [CrossRef]
- Apshingekar, P.P.; Aher, S.; Kelly, A.L.; Brown, E.C.; Paradkar, A. Synthesis of Caffeine/Maleic Acid Co-Crystal by Ultrasound-Assisted Slurry Co-Crystallization. J. Pharm. Sci. 2017, 106, 66–70. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Hong, C.; Yao, Y.; Shen, H.; Ji, G.; Li, G.; Xie, Y. Development of a Pharmaceutical Cocrystal with Solution Crystallization Technology: Preparation, Characterization, and Evaluation of Myricetin-Proline Cocrystals. Eur. J. Pharm. Biopharm. 2016, 107, 151–159. [Google Scholar] [CrossRef]
- Pantwalawalkar, J.; More, H.; Bhange, D.; Patil, U.; Jadhav, N. Novel Curcumin Ascorbic Acid Cocrystal for Improved Solubility. J. Drug Deliv. Sci. Technol. 2021, 61, 102233. [Google Scholar] [CrossRef]
- Li, Z.; Matzger, A.J. Influence of Coformer Stoichiometric Ratio on Pharmaceutical Cocrystal Dissolution: Three Cocrystals of Carbamazepine/4-Aminobenzoic Acid. Mol. Pharm. 2016, 13, 990–995. [Google Scholar] [CrossRef] [Green Version]
- Chow, S.F.; Shi, L.; Ng, W.W.; Leung, K.H.Y.; Nagapudi, K.; Sun, C.C.; Chow, A.H.L. Kinetic Entrapment of a Hidden Curcumin Cocrystal with Phloroglucinol. Cryst. Growth Des. 2014, 14, 5079–5089. [Google Scholar] [CrossRef]
- Desai, H.; Rao, L.; Amin, P. Carbamazepine Cocrystals by Solvent Evaporation Technique: Formulation and Characterization Studies. Am. J. PharmTech Res. 2014, 4, 479–493. [Google Scholar]
- Winantari, A.N.; Setyawan, D.; Siswodihardjo, S.; Soewandhi, S.N. Cocrystallization Acyclovir-Succinic Acid Using Solvent Evaporation Methods. Asian J. Pharm. Clin. Res. 2017, 10, 91. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Rohani, S. Cocrystals of Acyclovir with Promising Physicochemical Properties. J. Pharm. Sci. 2015, 104, 98–105. [Google Scholar] [CrossRef]
- Pan, X.; Zheng, Y.; Chen, R.; Qiu, S.; Chen, Z.; Rao, W.; Chen, S.; You, Y.; Lü, J.; Xu, L.; et al. Cocrystal of Sulfamethazine and P-Aminobenzoic Acid: Structural Establishment and Enhanced Antibacterial Properties. Cryst. Growth Des. 2019, 19, 2455–2460. [Google Scholar] [CrossRef]
- Nechipadappu, S.K.; Trivedi, D.R. Cocrystal of Nutraceutical Sinapic Acid with Active Pharmaceutical Ingredients Ethenzamide and 2-Chloro-4-Nitrobenzoic Acid: Equilibrium Solubility and Stability Study. J. Mol. Struct. 2018, 1171, 898–905. [Google Scholar] [CrossRef]
- Xuan, B.; Wong, S.N.; Zhang, Y.; Weng, J.; Tong, H.H.Y.; Wang, C.; Sun, C.C.; Chow, S.F. Extended Release of Highly Water Soluble Isoniazid Attained through Cocrystallization with Curcumin. Cryst. Growth Des. 2020, 20, 1951–1960. [Google Scholar] [CrossRef]
- Darwish, S.; Zeglinski, J.; Krishna, G.R.; Shaikh, R.; Khraisheh, M.; Walker, G.M.; Croker, D.M. A New 1:1 Drug-Drug Cocrystal of Theophylline and Aspirin: Discovery, Characterization, and Construction of Ternary Phase Diagrams. Cryst. Growth Des. 2018, 18, 7526–7532. [Google Scholar] [CrossRef] [Green Version]
- do Amaral, L.H.; do Carmo, F.A.; Amaro, M.I.; de Sousa, V.P.; da Silva, L.C.R.P.; de Almeida, G.S.; Rodrigues, C.R.; Healy, A.M.; Cabral, L.M. Development and Characterization of Dapsone Cocrystal Prepared by Scalable Production Methods. AAPS PharmSciTech 2018, 19, 2687–2699. [Google Scholar] [CrossRef]
- Bag, P.P.; Patni, M.; Malla Reddy, C. A Kinetically Controlled Crystallization Process for Identifying New Co-Crystal Forms: Fast Evaporation of Solvent from Solutions to Dryness. CrystEngComm 2011, 13, 5650–5652. [Google Scholar] [CrossRef]
- Wood, B.; Girard, K.P.; Polster, C.S.; Croker, D.M. Progress to Date in the Design and Operation of Continuous Crystallization Processes for Pharmaceutical Applications. Org. Process Res. Dev. 2019, 23, 122–144. [Google Scholar] [CrossRef]
- Gao, Z.; Rohani, S.; Gong, J.; Wang, J. Recent Developments in the Crystallization Process: Toward the Pharmaceutical Industry. Engineering 2017, 3, 343–353. [Google Scholar] [CrossRef]
- Trampuž, M.; Teslić, D.; Likozar, B. Crystal-Size Distribution-Based Dynamic Process Modelling, Optimization, and Scaling for Seeded Batch Cooling Crystallization of Active Pharmaceutical Ingredients (API). Chem. Eng. Res. Des. 2021, 165, 254–269. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Chow, P.S.; Tan, R.B.H. Operating Regions in Cooling Cocrystallization of Caffeine and Glutaric Acid in Acetonitrile. Cryst. Growth Des. 2010, 10, 2382–2387. [Google Scholar] [CrossRef]
- He, G.; Chow, P.S.; Tan, R.B.H. Investigating the Intermolecular Interactions in Concentration-Dependent Solution Cocrystallization of Caffeine and p -Hydroxybenzoic Acid. Cryst. Growth Des. 2010, 10, 3763–3769. [Google Scholar] [CrossRef]
- Sathisaran, I.; Dalvi, S.V. Investigating Cocrystallization of Carbamazepine with Structurally Compatible Coformers: New Cocrystal and Eutectic Phases with Enhanced Dissolution. AAPS PharmSciTech 2021, 22, 29. [Google Scholar] [CrossRef]
- Engku Mat Nasir, E.N.; Rahman, F.A.; Abd Rahim, S.; Edros, R.Z.; Anuar, N. Crystallisation Parameters Effect on the Particle Size Distribution (PSD) of Carbamazepine-Saccharin (CBZ-SAC) Co-Crystals in Batch Cooling Crystallisation. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2020; Volume 736, p. 022109. [Google Scholar] [CrossRef]
- Kitamura, M. Controlling Factor of Polymorphism in Crystallization Process. J. Cryst. Growth 2002, 237–239, 2205–2214. [Google Scholar] [CrossRef]
- Parambil, J.V.; Schaepertoens, M.; Williams, D.R.; Heng, J.Y.Y. Effects of Oscillatory Flow on the Nucleation and Crystallization of Insulin. Cryst. Growth Des. 2011, 11, 4353–4359. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.H.; Yu, T.; Lee, T.; Kim, W.S. Cocrystallization of Caffeine-Maleic Acid in a Batchelor Vortex Flow. Cryst. Growth Des. 2020, 20, 1618–1627. [Google Scholar] [CrossRef]
- Chen, T.H.; Yeh, K.L.; Chen, C.W.; Lee, H.L.; Hsu, Y.C.; Lee, T. Mixing Effect on Stoichiometric Diversity in Benzoic Acid-Sodium Benzoate Cocrystals. Cryst. Growth Des. 2019, 19, 1576–1583. [Google Scholar] [CrossRef]
- Hickey, M.B.; Peterson, M.L.; Scoppettuolo, L.A.; Morrisette, S.L.; Vetter, A.; Guzmán, H.; Remenar, J.F.; Zhang, Z.; Tawa, M.D.; Haley, S. Performance Comparison of a Co-Crystal of Carbamazepine with Marketed Product. Eur. J. Pharm. Biopharm. 2007, 67, 112–119. [Google Scholar] [CrossRef]
- Kim, K.-J. Industrial Crystallization. Chem. Eng. Technol. 2016, 39, 1212. [Google Scholar] [CrossRef]
- Kudo, S.; Takiyama, H. Solubility Determination for Carbamazepine and Saccharin in Methanol/Water Mixed Solvent: Basic Data for Design of Cocrystal Production by Antisolvent Crystallization. J. Chem. Eng. Data 2018, 63, 451–458. [Google Scholar] [CrossRef]
- Lange, L.; Heisel, S.; Sadowski, G. Predicting the Solubility of Pharmaceutical Cocrystals in Solvent/Anti-Solvent Mixtures. Molecules 2016, 21, 593. [Google Scholar] [CrossRef] [Green Version]
- Veith, H.; Schleinitz, M.; Schauerte, C.; Sadowski, G. Thermodynamic Approach for Co-Crystal Screening. Cryst. Growth Des. 2019, 19, 3253–3264. [Google Scholar] [CrossRef]
- Loschen, C.; Klamt, A. Cocrystal Ternary Phase Diagrams from Density Functional Theory and Solvation Thermodynamics. Cryst. Growth Des. 2018, 18, 5600–5608. [Google Scholar] [CrossRef]
- Wang, I.-C.; Lee, M.-J.; Sim, S.-J.; Kim, W.-S.; Chun, N.-H.; Choi, G.J. Anti-Solvent Co-Crystallization of Carbamazepine and Saccharin. Int. J. Pharm. 2013, 450, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-J.; Wang, I.-C.; Kim, M.-J.; Kim, P.; Song, K.-H.; Chun, N.-H.; Park, H.-G.; Choi, G.J. Controlling the Polymorphism of Carbamazepine-Saccharin Cocrystals Formed during Antisolvent Cocrystallization Using Kinetic Parameters. Korean J. Chem. Eng. 2015, 32, 1910–1917. [Google Scholar] [CrossRef]
- Leung, D.H.; Lohani, S.; Ball, R.G.; Canfield, N.; Wang, Y.; Rhodes, T.; Bak, A. Two Novel Pharmaceutical Cocrystals of a Development Compound—Screening, Scale-up, and Characterization. Cryst. Growth Des. 2012, 12, 1254–1262. [Google Scholar] [CrossRef]
- Chun, N.H.; Lee, M.J.; Song, G.H.; Chang, K.Y.; Kim, C.S.; Choi, G.J. Combined Anti-Solvent and Cooling Method of Manufacturing Indomethacin-Saccharin (IMC-SAC) Co-Crystal Powders. J. Cryst. Growth 2014, 408, 112–118. [Google Scholar] [CrossRef]
- Fontana, F.; Figueiredo, P.; Zhang, P.; Hirvonen, J.T.; Liu, D.; Santos, H.A. Production of Pure Drug Nanocrystals and Nano Co-Crystals by Confinement Methods. Adv. Drug Deliv. Rev. 2018, 131, 3–21. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.G.Z.; Henry, R.F.; Borchardt, T.B.; Lou, X. Efficient Co-Crystal Screening Using Solution-Mediated Phase Transformation. J. Pharm. Sci. 2007, 96, 990–995. [Google Scholar] [CrossRef]
- Aitipamula, S.; Wong, A.B.H.; Chow, P.S.; Tan, R.B.H. Cocrystallization with Flufenamic Acid: Comparison of Physicochemical Properties of Two Pharmaceutical Cocrystals. CrystEngComm 2014, 16, 5793–5801. [Google Scholar] [CrossRef]
- Hong, C.; Xie, Y.; Yao, Y.; Li, G.; Yuan, X.; Shen, H. A Novel Strategy for Pharmaceutical Cocrystal Generation without Knowledge of Stoichiometric Ratio: Myricetin Cocrystals and a Ternary Phase Diagram. Pharm. Res. 2015, 32, 47–60. [Google Scholar] [CrossRef]
- Ahuja, D.; Ramisetty, K.A.; Sumanth, P.K.; Crowley, C.M.; Lusi, M.; Rasmuson, Å.C. Microwave Assisted Slurry Conversion Crystallization for Manufacturing of New Co-Crystals of Sulfamethazine and Sulfamerazine. CrystEngComm 2020, 22, 1381–1394. [Google Scholar] [CrossRef]
- Aher, S.; Dhumal, R.; Mahadik, K.; Paradkar, A.; York, P. Ultrasound Assisted Cocrystallization from Solution (USSC) Containing a Non-Congruently Soluble Cocrystal Component Pair: Caffeine/Maleic Acid. Eur. J. Pharm. Sci. 2010, 41, 597–602. [Google Scholar] [CrossRef]
- Soares, F.L.F.; Carneiro, R.L. In-Line Monitoring of Cocrystallization Process and Quantification of Carbamazepine-Nicotinamide Cocrystal Using Raman Spectroscopy and Chemometric Tools. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 180, 1–8. [Google Scholar] [CrossRef]
- Qu, H.; Jin, S.; Gong, J.; Du, S.; Jia, L.; Wu, S. Enhancing Stability and Formulation Capability of Fungicides by Cocrystallization through a Novel Multistep Slurry Conversion Process. Cryst. Growth Des. 2020, 20, 7356–7367. [Google Scholar] [CrossRef]
- Pawar, N.; Agrawal, S.; Methekar, R. Modeling, Simulation, and Influence of Operational Parameters on Crystal Size and Morphology in Semibatch Antisolvent Crystallization of α-Lactose Monohydrate. Cryst. Growth Des. 2018, 18, 4511–4521. [Google Scholar] [CrossRef]
- Rodrigues, M.; Lopes, J.; Guedes, A.; Sarraguça, J.; Sarraguça, M. Considerations on High-Throughput Cocrystals Screening by Ultrasound Assisted Cocrystallization and Vibrational Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117876. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.A.; Montgomery, S.E.; Gupta, R.B. Formation of Itraconazole/L-Malic Acid Cocrystals by Gas Antisolvent Cocrystallization. Powder Technol. 2013, 236, 122–131. [Google Scholar] [CrossRef]
- Wichianphong, N.; Charoenchaitrakool, M. Statistical Optimization for Production of Mefenamic Acid–Nicotinamide Cocrystals Using Gas Anti-Solvent (GAS) Process. J. Ind. Eng. Chem. 2018, 62, 375–382. [Google Scholar] [CrossRef]
- Pessoa, A.S.; Aguiar, G.P.S.; Vladimir Oliveira, J.; Bortoluzzi, A.J.; Paulino, A.; Lanza, M. Precipitation of Resveratrol-Isoniazid and Resveratrol-Nicotinamide Cocrystals by Gas Antisolvent. J. Supercrit. Fluids 2019, 145, 93–102. [Google Scholar] [CrossRef]
- Ribas, M.M.; Aguiar, G.P.S.; Muller, L.G.; Siebel, A.M.; Lanza, M.; Oliveira, J.V. Curcumin-Nicotinamide Cocrystallization with Supercritical Solvent (CSS): Synthesis, Characterization and in Vivo Antinociceptive and Anti-Inflammatory Activities. Ind. Crops Prod. 2019, 139, 111537. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Tiago, J.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Insight into the Mechanisms of Cocrystallization of Pharmaceuticals in Supercritical Solvents. Cryst. Growth Des. 2015, 15, 3175–3181. [Google Scholar] [CrossRef]
- Pando, C.; Cabañas, A.; Cuadra, I.A. Preparation of Pharmaceutical Co-Crystals through Sustainable Processes Using Supercritical Carbon Dioxide: A Review. RSC Adv. 2016, 6, 71134–71150. [Google Scholar] [CrossRef]
- MacEachern, L.; Kermanshahi-pour, A.; Mirmehrabi, M. Supercritical Carbon Dioxide for Pharmaceutical Co-Crystal Production. Cryst. Growth Des. 2020, 20, 6226–6244. [Google Scholar] [CrossRef]
- Long, B.; Ryan, K.M.; Padrela, L. From Batch to Continuous—New Opportunities for Supercritical CO2 Technology in Pharmaceutical Manufacturing. Eur. J. Pharm. Sci. 2019, 137, 104971. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, H.; Xu, J.; Wang, S.; Wang, H.; Yu, Z.; Zhao, L.; Zhu, C.; Sun, J. The Competition between Cocrystallization and Separated Crystallization Based on Crystallization from Solution. J. Appl. Crystallogr. 2019, 52, 769–776. [Google Scholar] [CrossRef]
- De Maere D’Aertrycke, J.B.; Payen, R.; Collard, L.; Robeyns, K.; Croker, D.; Leyssens, T. Enabling Cocrystallization of Challenging Systems: Passing through a Stable Cocrystal Solvate as a Pathway to Strenuous Cocrystal Forms. Cryst. Growth Des. 2020, 20, 2035–2043. [Google Scholar] [CrossRef]
- Mohammad, K.A.; Abd Rahim, S.; Abu Bakar, M.R. Kinetics and Nucleation Mechanism of Carbamazepine–Saccharin Co-Crystals in Ethanol Solution. J. Therm. Anal. Calorim. 2017, 130, 1663–1669. [Google Scholar] [CrossRef] [Green Version]
- Croker, D.M.; Davey, R.J.; Rasmuson, Å.C.; Seaton, C.C. Nucleation in the p -Toluenesulfonamide/Triphenylphosphine Oxide Co-Crystal System. Cryst. Growth Des. 2013, 13, 3754–3762. [Google Scholar] [CrossRef]
- Sheikh, A.Y.; Rahim, S.A.; Hammond, R.B.; Roberts, K.J. Scalable Solution Cocrystallization: Case of Carbamazepine-Nicotinamide I. CrystEngComm 2009, 11, 501–509. [Google Scholar] [CrossRef]
- Yu, Z.Q.; Chow, P.S.; Tan, R.B.H. Design Space for Polymorphic Co-Crystallization: Incorporating Process Model Uncertainty and Operational Variability. Cryst. Growth Des. 2014, 14, 3949–3957. [Google Scholar] [CrossRef]
- Weng, J.; Wong, S.N.; Xu, X.; Xuan, B.; Wang, C.; Chen, R.; Sun, C.C.; Lakerveld, R.; Kwok, P.C.L.; Chow, S.F. Cocrystal Engineering of Itraconazole with Suberic Acid via Rotary Evaporation and Spray Drying. Cryst. Growth Des. 2019, 19, 2736–2745. [Google Scholar] [CrossRef]
- MacEachern, L.A.; Walwyn-Venugopal, R.; Kermanshahi-pour, A.; Mirmehrabi, M. Ternary Phase Diagram Development and Production of Niclosamide-Urea Co-Crystal by Spray Drying. J. Pharm. Sci. 2020. [Google Scholar] [CrossRef]
- Walsh, D.; Serrano, D.R.; Worku, Z.A.; Norris, B.A.; Healy, A.M. Production of Cocrystals in an Excipient Matrix by Spray Drying. Int. J. Pharm. 2018, 536, 467–477. [Google Scholar] [CrossRef]
- Pawar, N.; Agrawal, S.; Methekar, R. Continuous Antisolvent Crystallization of α-Lactose Monohydrate: Impact of Process Parameters, Kinetic Estimation, and Dynamic Analysis. Org. Process Res. Dev. 2019, 23, 2394–2404. [Google Scholar] [CrossRef]
- Darmali, C.; Mansouri, S.; Yazdanpanah, N.; Woo, M.W. Mechanisms and Control of Impurities in Continuous Crystallization: A Review. Ind. Eng. Chem. Res. 2019, 58, 1463–1479. [Google Scholar] [CrossRef]
- Pu, S.; Hadinoto, K. Continuous Crystallization as a Downstream Processing Step of Pharmaceutical Proteins: A Review. Chem. Eng. Res. Des. 2020, 160, 89–104. [Google Scholar] [CrossRef]
- Orehek, J.; Teslić, D.; Likozar, B. Continuous Crystallization Processes in Pharmaceutical Manufacturing: A Review. Org. Process Res. Dev. 2021, 25, 16–42. [Google Scholar] [CrossRef]
- Kelly, A.L.; Gough, T.; Dhumal, R.S.; Halsey, S.A.; Paradkar, A. Monitoring Ibuprofen-Nicotinamide Cocrystal Formation during Solvent Free Continuous Cocrystallization (SFCC) Using near Infrared Spectroscopy as a PAT Tool. Int. J. Pharm. 2012, 426, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Moradiya, H.G.; Islam, M.T.; Halsey, S.; Maniruzzaman, M.; Chowdhry, B.Z.; Snowden, M.J.; Douroumis, D. Continuous Cocrystallisation of Carbamazepine and Trans-Cinnamic Acid via Melt Extrusion Processing. CrystEngComm 2014, 16, 3573–3583. [Google Scholar] [CrossRef]
- Chabalenge, B.; Korde, S.; Kelly, A.L.; Neagu, D.; Paradkar, A. Understanding Matrix-Assisted Continuous Co-Crystallization Using a Data Mining Approach in Quality by Design (QbD). Cryst. Growth Des. 2020, 20, 4540–4549. [Google Scholar] [CrossRef]
- Shaikh, R.; Walker, G.M.; Croker, D.M. Continuous, Simultaneous Cocrystallization and Formulation of Theophylline and 4-Aminobenzoic Acid Pharmaceutical Cocrystals Using Twin Screw Melt Granulation. Eur. J. Pharm. Sci. 2019, 137, 104981. [Google Scholar] [CrossRef]
- Lee, T.; Chen, H.R.; Lin, H.Y.; Lee, H.L. Continuous Co-Crystallization As a Separation Technology: The Study of 1:2 Co-Crystals of Phenazine–Vanillin. Cryst. Growth Des. 2012, 12, 5897–5907. [Google Scholar] [CrossRef]
- McGlone, T.; Briggs, N.E.B.; Clark, C.A.; Brown, C.J.; Sefcik, J.; Florence, A.J. Oscillatory Flow Reactors (OFRs) for Continuous Manufacturing and Crystallization. Org. Process Res. Dev. 2015, 19, 1186–1202. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Raval, V.; Briggs, N.E.B.; Bhardwaj, R.M.; McGlone, T.; Oswald, I.D.H.; Florence, A.J. From Discovery to Scale-up: α-Lipoic Acid: Nicotinamide Co-Crystals in a Continuous Oscillatory Baffled Crystalliser. CrystEngComm 2014, 16, 5769–5780. [Google Scholar] [CrossRef] [Green Version]
- Nishimaru, M.; Nakasa, M.; Kudo, S.; Takiyama, H. Operation Condition for Continuous Anti-Solvent Crystallization of CBZ-SAC Cocrystal Considering Deposition Risk of Undesired Crystals. J. Cryst. Growth 2017, 470, 89–93. [Google Scholar] [CrossRef]
- Svoboda, V.; Macfhionnghaile, P.; McGinty, J.; Connor, L.E.; Oswald, I.D.H.; Sefcik, J. Continuous Cocrystallization of Benzoic Acid and Isonicotinamide by Mixing-Induced Supersaturation: Exploring Opportunities between Reactive and Antisolvent Crystallization Concepts. Cryst. Growth Des. 2017, 17, 1902–1909. [Google Scholar] [CrossRef] [Green Version]
- Holaň, J.; Ridvan, L.; Billot, P.; Štěpánek, F. Design of Co-Crystallization Processes with Regard to Particle Size Distribution. Chem. Eng. Sci. 2015, 128, 36–43. [Google Scholar] [CrossRef]
- Erriguible, A.; Neurohr, C.; Revelli, A.L.; Laugier, S.; Fevotte, G.; Subra-Paternault, P. Cocrystallization Induced by Compressed CO2 as Antisolvent: Simulation of a Batch Process for the Estimation of Nucleation and Growth Parameters. J. Supercrit. Fluids 2015, 98, 194–203. [Google Scholar] [CrossRef]
- Neurohr, C.; Erriguible, A.; Laugier, S.; Subra-Paternault, P. Challenge of the Supercritical Antisolvent Technique SAS to Prepare Cocrystal-Pure Powders of Naproxen-Nicotinamide. Chem. Eng. J. 2016, 303, 238–251. [Google Scholar] [CrossRef]
- Mishra, V.; Thakur, S.; Patil, A.; Shukla, A. Quality by Design (QbD) Approaches in Current Pharmaceutical Set-Up. Expert Opin. Drug Deliv. 2018, 15, 737–758. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.L.; Pataki, H.; Marosi, G.; Meemken, F.; Hungerbühler, K.; Baiker, A.; Tummala, S.; Glennon, B.; Kuentz, M.; Steele, G.; et al. Assessment of Recent Process Analytical Technology (PAT) Trends: A Multiauthor Review. Org. Process Res. Dev. 2015, 19, 3–62. [Google Scholar] [CrossRef]
- Powell, K.A.; Croker, D.M.; Rielly, C.D.; Nagy, Z.K. PAT-Based Design of Agrochemical Co-Crystallization Processes: A Case-Study for the Selective Crystallization of 1:1 and 3:2 Co-Crystals of p-Toluenesulfonamide/Triphenylphosphine Oxide. Chem. Eng. Sci. 2016, 152, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Chavan, R.B.; Thipparaboina, R.; Yadav, B.; Shastri, N.R. Continuous Manufacturing of Co-Crystals: Challenges and Prospects. Drug Deliv. Transl. Res. 2018, 8, 1726–1739. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.; Ganesh, S.; Moreno, M.; Bommireddy, Y.; Gonzalez, M.; Reklaitis, G.V.; Nagy, Z.K. A Perspective on Quality-by-Control (QbC) in Pharmaceutical Continuous Manufacturing. Comput. Chem. Eng. 2019, 125, 216–231. [Google Scholar] [CrossRef]

| Solution Cocrystallization | Solid-State Cocrystallization |
|---|---|
|
|
|
|
|
|
| Advantages | Advantages |
|
|
|
|
|
|
| Disadvantages |
|
|
|
|
| Disadvantages |
|
| |
|
| Reference | Model Compound | Coformer | Synthesis Method | Remark |
|---|---|---|---|---|
| Yu et al., (2021) [27] | Urea | Succinic acid | Cooling | Achieved desired polymorphic form |
| Huang et al., (2019) [28] | Theophylline | Benzoic acid | Cooling | - |
| Thakor et al., (2020) [29] | Carbamazepine | Nicotinamide | Antisolvent | Synthesized nano-sized cocrystals |
| Yang et al., (2020) [30] | 1,3,5-Trinitrobenzene | 1-methyl-2,4-dinitroimidazole | Antisolvent | Production of energetic cocrystals |
| Guo et al., (2020) [31] | Nicorandil | 1-hydroxy-2-naphthoic acid and salicylic acid | Evaporation | Enhanced chemical stability and dissolution rate of cocrystals |
| Wu et al., (2020) [32] | Quercetin | Nicotinamide | Evaporation | Higher dissolution rate and improved bioavailability |
| Luo et al., (2018) [33] | Naringenin | Isonicotinamide, picolinic acid, and betaine | Slurry method | Improved equilibrium solubility of cocrystals |
| Inam et al., (2018) [34] | Ticagrelor | Nicotinamide | Slurry method | Improved solubility and dissolution rate of cocrystals |
| Cuadra et al., (2018) [35] | Carbamazepine | Saccharin | Supercritical fluid | Higher dissolution efficiency of cocrystal. |
| Apshingekar et al., (2017) [36] | Caffeine | Maleic acid | Ultrasound-assisted | Improved dissolution rates of the cocrystals. |
| Liu et al., (2016) [37] | Myricetin | Proline | Ultrasound-assisted | Improved dissolution rate and bioavailability |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawar, N.; Saha, A.; Nandan, N.; Parambil, J.V. Solution Cocrystallization: A Scalable Approach for Cocrystal Production. Crystals 2021, 11, 303. https://doi.org/10.3390/cryst11030303
Pawar N, Saha A, Nandan N, Parambil JV. Solution Cocrystallization: A Scalable Approach for Cocrystal Production. Crystals. 2021; 11(3):303. https://doi.org/10.3390/cryst11030303
Chicago/Turabian StylePawar, Nitin, Anindita Saha, Neelesh Nandan, and Jose V. Parambil. 2021. "Solution Cocrystallization: A Scalable Approach for Cocrystal Production" Crystals 11, no. 3: 303. https://doi.org/10.3390/cryst11030303
APA StylePawar, N., Saha, A., Nandan, N., & Parambil, J. V. (2021). Solution Cocrystallization: A Scalable Approach for Cocrystal Production. Crystals, 11(3), 303. https://doi.org/10.3390/cryst11030303