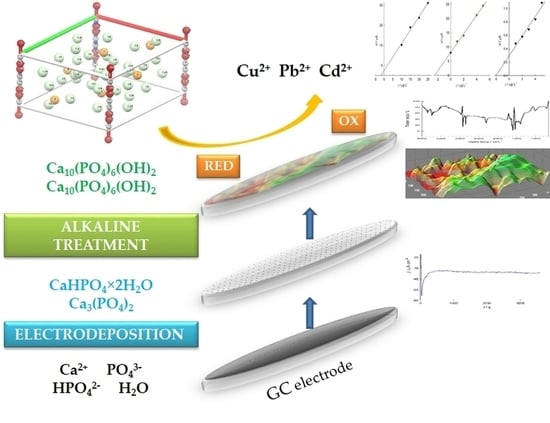
The Preparation, Morphological Characterization and Possible Electroanalytical Application of a Hydroxyapatite-Modified Glassy Carbon Electrode
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Electrodeposition of Ca/P Phases on the GC Electrode and Surface Morphology of the HAp Coating on the GC Electrode
3.1.1. Deposition
3.1.2. Atomic Force Microscopy (AFM)
3.1.3. Scanning Electron Microscopy (SEM)
3.1.4. Fourier-Transform Infrared Spectroscopy (FTIR)
- (1).
- The strong characteristic band in the 3400–3900 cm−1 frequency range and at 2310 cm−1 is assigned to O−H stretching.
- (2).
- The peaks at 2848 and 2919 cm−1 of C–H stretching from the methyl moiety (−CH2), which originated from organic impurities, suggest a noncovered GC surface.
- (3).
- The peak at 1623 cm−1 was due to adsorbed atmospheric CO2, and the peaks at 1540 and 1380 cm−1 can be attributed to the vibration of traces of organic substances on the surface or the vibration of a CO32− group.
- (4).
- The most prominent peaks at 1015 cm−1 are due to the asymmetrical P−O stretching mode of HAp in phosphate.
- (5).
- The slight shoulders at 961 and 560 cm−1 and the peak at 604 cm−1 are ascribed to the P−O bending mode from phosphate.
3.1.5. Electrochemical Impedance Spectroscopy (EIS) Studies of HAp/GC
3.1.6. Electrochemical Methods
4. Discussion
4.1. Analytical Applicability
4.2. Analytical Application of HAp/GC Electrode in Real Samples
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di, N.; Zhang, K.; Hladun, K.R.; Rust, M.; Chen, Y.-F.; Zhu, Z.-Y.; Liu, T.X.; Trumble, J.T. Joint effects of cadmium and copper on Apis mellifera foragers and larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 237, 108839. [Google Scholar] [CrossRef]
- Sousa, M.; Benson, B.; Welty, C.; Price, D.; Thirkill, R.; Erickson, W.; Cummings, M.; Dunnivant, F. Atmospheric deposition of coal-related pollutants in the Pacific Northwest of the United States from 1950 to 2016. Environ. Tox. Chem. 2019, 39, 335–342. [Google Scholar] [CrossRef]
- Zhou, Q. Interactions between heavy metals and nitrogen fertilizers applied to soil-vegetable systems. Bull. Environ. Contam. Tox. 2003, 71, 338–344. [Google Scholar] [CrossRef]
- Herawati, N.; Suzuki, S.; Hayashi, K.; Rivai, I.F.; Koyama, H. Cadmium, Copper, and Zinc Levels in Rice and Soil of Japan, Indonesia, and China by Soil Type. Bull. Environ. Contam. Toxicol. 2000, 64, 33–39. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Peña-Crecente, R.M. The use of honeybees and honey as environmental bioindicators for metals and radionuclides: A review. Environ. Rev. 2017, 25, 463–480. [Google Scholar] [CrossRef]
- Conti, M.E.; Botrè, F. Honeybees and Their Products as Potential Bioindicators of Heavy Metals Contamination. Environ. Monit. Assess 2001, 69, 267–282. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Saf. 2017, 16, 1072–1100. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Sikandar, F.; Ullah, I.; Shah, A.; Ud-Din Khan, S.; Ali Rana, U.; McCoy, T. Spectrophotometric Determination of Trace Elements in various Honey Samples, Collected from different Environments. Food. Nutr. Res. 2014, 2, 532–538. [Google Scholar] [CrossRef] [Green Version]
- López-García, I.; Viñas, P.; Blanco, C.; Hernández-Córdoba, M. Fast determination of calcium, magnesium and zinc in honey using continuous flow flame atomic absorption spectrometry. Talanta 1999, 49, 597–602. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, A.A.; Ghandour, A.A.M.M.; Mahran, H.A. Evaluation of lead, cadmium and copper concentrations in bee honey. Am. J. Appl. Sci. 2010, 7, 315–322. [Google Scholar] [CrossRef]
- Chen, L.Z.; Rui, Y.K.; Zhao, J.; Ye, Z.H.; Xue, X.F.; Wang, P. Application of ICP-MS to detection of trace elements and heavy metals in different kinds of honey. Guang Pu Xue Yu Guang Pu Fen Xi/Spectrosc. Spect. Anal. 2008, 28, 1403–1405. [Google Scholar]
- Ioannidou, M.D.; Zachariadis, G.A.; Anthemidis, A.N.; Stratis, J.A. Direct determination of toxic metals in honey and sugar using ICP-AES. Talanta 2005, 65, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, P.; Naithani, P.; Tiwari, J.K. Determination of Heavy Metals in Honey Samples from Sub—Montane and Montane Zones of Garhwal Himalaya (India). World J. Pharm. Pharm. Sci. 2016, 5, 812–819. [Google Scholar] [CrossRef]
- Alghamdi, A.H. Applications of stripping voltammetric techniques in food analysis. Arab. J. Chem. 2010, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sanna, G.; Pilo, I.M.; Piu, P.C.; Tapparo, A.; Seeber, R. Determination of heavy metals in honey by anodic stripping voltammetry at microelectrodes. Anal. Chim. Acta 2000, 415, 165–173. [Google Scholar] [CrossRef]
- Sochr, J.; Machková, M.; Machyňák, Ľ.; Čacho, F.; Švorc, Ľ. Heavy metals determination using various bismuth film modified carbon-based electrodes. Acta Chim. Slov. 2016, 9, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Pierini, G.D.; Pistonesi, M.F.; Di Nezio, M.S.; Centurión, M.E. A pencil-lead bismuth film electrode and chemometric tools for simultaneous determination of heavy metals in propolis samples. Microchem. J. 2016, 125, 266–272. [Google Scholar] [CrossRef]
- Santos Nascimento, A.; Marchini, L.; Carvalho, C.; Araújo, D.; Silveira, T.; Olinda, R. Determining the Levels of Trace Elements Cd, Cu, Pb and Zn in Honey of Stingless Bee (Hymenoptera: Apidae ) Using Voltammetry. Food Nutr. Sci. 2015, 6, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Vladislavić, N.; Rončević, I.Š.; Buzuk, M.; Buljac, M.; Drventić, I. Electrochemical/chemical synthesis of hydroxyapatite on glassy carbon electrode for electroanalytical determination of cysteine. J. Solid State Electrochem. 2021, 25, 841–857. [Google Scholar] [CrossRef]
- Malmberg, P.; Bigdeli, N.; Jensen, J.; Nygren, H. Formation of hydroxyapatite on titanium implants in vivo precedes bone-formation during healing. Biointerphases 2017, 12, 041002. [Google Scholar] [CrossRef] [Green Version]
- Nygren, H.; Bigdeli, N.; Ilver, L.; Malmberg, P. Mg-corrosion, hydroxyapatite, and bone healing. Biointerphases 2017, 12, 02C407. [Google Scholar] [CrossRef] [Green Version]
- Skwarek, E.; Janusz, W.; Sternik, D. The influence of the hydroxyapatite synthesis method on the electrochemical, surface and adsorption properties of hydroxyapatite. Adsorp. Sci. Technol. 2017, 35, 507–518. [Google Scholar] [CrossRef] [Green Version]
- Kanchana, P.; Sekar, C. Development of electrochemical folic acid sensor based on hydroxyapatite nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.F.; Johnson, S.; Kumar, D.; Jelinek, M.; Chrisey, D.B.; Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mihailescu, I.N. Pulsed laser deposition of hydroxyapatite thin films’. Mater. Sci. Eng. C 2007, 27, 484–494. [Google Scholar] [CrossRef]
- Sun, L.M.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570. [Google Scholar] [CrossRef]
- Hamdi, M.; Hakamata, S.; Ektessabi, A.M. Coating of hydroxyapatite thin film by simultaneous vapor deposition. Thin Solid Films 2000, 377–378, 484. [Google Scholar] [CrossRef]
- Sun, L.M.; Berndt, C.C.; Khor, K.A.; Cheang, H.N.; Gross, K.A. Surface characteristics and dissolution behavior of plasma-sprayed hydroxyapatite coating. J. Biomed. Mater. Res. 2002, 62, 228. [Google Scholar] [CrossRef]
- Jin, B.; Shao, C.; Wang, Y.; Mu, Z.; Liu, Z.; Tang, R. Anisotropic Epitaxial Behavior in the Amorphous Phase-Mediated Hydroxyapatite Crystallization Process: A New Understanding of Orientation Control. J. Phys. Chem. Lett. 2019, 10, 7611–7616. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.Y.; Hamdi, M.; Sopyan, I.; Teng, W.D. The influence of Ca/P ratio on the properties of hydroxyapatite bioceramics. In Proceedings Volume 6423, International Conference on Smart Materials and Nanotechnology in Engineering; International Society for Optics and Photonics: Bellingham, WA, USA, 2007; p. 64233A. [Google Scholar] [CrossRef]
- Du, L.-W.; Bian, S.; Gou, B.-D.; Jiang, Y.; Huang, J.; Gao, Y.-X.; Zhao, Y.-D.; Wen, W.; Zhang, T.-L.; Wang, K. Structure of Clusters and Formation of Amorphous Calcium Phosphate and Hydroxyapatite: From the Perspective of Coordination Chemistry. Cryst. Growth Des. 2013, 13, 3103–3109. [Google Scholar] [CrossRef]
- Rončević, I.Š.; Grubač, Z.; Metikoš-Huković, M. Electrodeposition of hydroxyapatite coating on AZ91D magnesium alloy for biomaterial application. Int. J. Electrochem. Sci. 2014, 9, 5907–5923. [Google Scholar]
- Vladislavić, N.; Buzuk, M.; Brinić, S.; Buljac, M.; Bralić, M. Morphological characterization of ex situ prepared bismuth film electrodes and their application in electroanalytical determination of the biomolecules. J. Solid State Electrochem. 2016, 20, 2241–2250. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Zeng, X.; Liu, Y.; Liu, X.; Wei, W.; Luo, S. Simultaneous determination of ultra-trace lead and cadmium at a hydroxyapatite-modified carbon ionic liquid electrode by square-wave stripping voltammetry. Sens. Actuators B Chem. 2009, 139, 604–610. [Google Scholar] [CrossRef]
- Pan, D.; Wang, Y.; Chen, Z.; Lou, T.; Qin, W. Nanomaterial/Ionophore-Based Electrode for Anodic Stripping Voltammetric Determination of Lead:An Electrochemical Sensing Platform toward Heavy Metals. Anal. Chem. 2009, 81, 5088–5094. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Wang, Y.; Chen, Z.; Yin, T.; Qin, W. Fabrication and characterization of carbon nanotube-hydroxyapatite nanocomposite: Application to anodic stripping voltammetric determination of cadmium. Electroanalysis 2009, 21, 944–952. [Google Scholar] [CrossRef]
- Khan, A.A.A.; Abdullah, M.A. Bismuth-modified hydroxyapatite carbon electrode for simultaneous in-situ cadmium and lead analysis. Int. J. Electrochem. Sci. 2013, 8, 195–203. [Google Scholar]
- El Mhammedi, M.A.; Achak, M.; Bakasse, M. Evaluation of a platinum electrode modified with hydroxyapatite in the lead(II) determination in a square wave voltammetric procedure. Arabian J. Chem. 2013, 6, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Mortada, W.I.; Kenawy, I.M.M.; Abdelghany, A.M.; Ismail, A.M.; Donia, A.F.; Nabieh, K.A. Determination of Cu2+, Zn2+ and Pb2+ in biological and food samples by FAAS after preconcentration with hydroxyapatite nanorods originated from eggshell. Mater. Sci. Eng. C 2015, 52, 288–296. [Google Scholar] [CrossRef]
- Raizda, P.; Gautam, S.; Priya, B.; Singh, P. Preparation and photocatalytic activity of hydroxyapatite supported BiOCl nanocomposite for oxytetracyline removal. Adv. Mater. Lett. 2016, 7, 312–318. [Google Scholar] [CrossRef]
- Boukamp, B.A. A Nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ion. 1986, 20, 31–44. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing, Solid Materials and Systems; John Wiley & Sons Inc.: New York, NY, USA, 1987; p. 301. ISBN 10:0471831220. [Google Scholar]
- Shimizu, K.; Sepunaru, L.; Compton, R.G. Innovative catalyst design for the oxygen reduction reaction for fuel cells. Chem. Sci. 2016, 7, 3364–3369. [Google Scholar] [CrossRef] [Green Version]









| Electrode Modification/Substrate | Electroanalytical Methods | Analytes | Linear Range (µg/L) | Analyzed Samples | LOD (µg/L) | Ref. |
|---|---|---|---|---|---|---|
| HAp–CILE/CP 1 | CV SWASV | Pb Cd | 0.11–0.121 | Wastewater sample | 0.041 0.056 | 2009 [33] |
| nHAp/ionophore/Nafion/GCE 2 | CV ASDPV | Pb | 1 to 166 | Real water sample | 0.21 | 2009 [34] |
| CNT–HAp/GCE 3 | CV DPV | Cd | 2.25–33,723 | Real tap water sample | 0.45 | 2009 [35] |
| Bi/HAp–CME 4 | CV SWASV | Pb Cd | Up to 150 | Spiked lake water sample | 5 5 | 2013 [36] |
| HAp/Pt 5 | CV SWASV | Pb | 4.14–2277 | River water samples | 4.2 | 2013 [37] |
| FAAS 6 | FAAS | Cu Zn Pb | 0.72 0.55 0.512 | 2015 [38] | ||
| HAp/GCE 7 | CV SWASV | Cu Pb Cd | 10–1000 10–1000 1–100 | Real sugar and honey samples | 2.0 10.0 0.9 | 2021 This work |
| Cu2+ | Pb2+ | Cd2+ | |
|---|---|---|---|
| Supporting electrolyte | 0.2 M KNO3 | 0.2 M KNO3 | 0.2 M KNO3 |
| pH | 4 | 4 | 4 |
| Accumulation potential | −500 mV | −1000 mV | −1200 mV |
| Accumulation time | 300 s | 240 s | 120 s |
| Frequency | 50 Hz | 50 Hz | 50 Hz |
| Potential increment | 5 mV | 5 mV | 5 mV |
| Pulse height | 50 mV | 50 mV | 20 mV |
| Stripping potential | ~100 mV | ~400 mV | ~700 mV |
| Concentration region with linear responses | 10 µg L−1–100 µg 100–1000 µg L−1 | 10 µg L−1–100 µg 100–1000 µg L−1 | 1.0 µg L−1–10 µg 10–100 µg L−1 |
| Correlation coefficient, R2 | 0.998 0.994 | 0.989 0.993 | 0.986 0.994 |
| Sensitivity | 0.03 µA µg L−1 0.04 µA µg L−1 | 0.09 µA µg L−1 0.19 µA µg L−1 | 0.11 µA µg L−1 0.03 µA µg L−1 |
| Real Sample | Honey 1 | Honey 2 | Sugar | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Pb | Cd | Cu | Pb | Cd | Cu | Pb | Cd | |
| µg kg−1 | 1775 ± 47 | 587.5 ± 22.1 | 64.3 ± 1.7 | 373.0 ± 12.0 | 4410 ± 66 | 287.4 ± 12.6 | 693.7 ± 23.5 | 823.7 ± 13.5 | 360.0 ± 2.7 |
| Maximum Qty Allowed | 2000 | 300 | 300 | 2000 | 300 | 300 | 1000 | 500 | 500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Škugor Rončević, I.; Buzuk, M.; Buljac, M.; Vladislavić, N. The Preparation, Morphological Characterization and Possible Electroanalytical Application of a Hydroxyapatite-Modified Glassy Carbon Electrode. Crystals 2021, 11, 772. https://doi.org/10.3390/cryst11070772
Škugor Rončević I, Buzuk M, Buljac M, Vladislavić N. The Preparation, Morphological Characterization and Possible Electroanalytical Application of a Hydroxyapatite-Modified Glassy Carbon Electrode. Crystals. 2021; 11(7):772. https://doi.org/10.3390/cryst11070772
Chicago/Turabian StyleŠkugor Rončević, Ivana, Marijo Buzuk, Maša Buljac, and Nives Vladislavić. 2021. "The Preparation, Morphological Characterization and Possible Electroanalytical Application of a Hydroxyapatite-Modified Glassy Carbon Electrode" Crystals 11, no. 7: 772. https://doi.org/10.3390/cryst11070772
APA StyleŠkugor Rončević, I., Buzuk, M., Buljac, M., & Vladislavić, N. (2021). The Preparation, Morphological Characterization and Possible Electroanalytical Application of a Hydroxyapatite-Modified Glassy Carbon Electrode. Crystals, 11(7), 772. https://doi.org/10.3390/cryst11070772