Crystal Chemical Relations in the Shchurovskyite Family: Synthesis and Crystal Structures of K2Cu[Cu3O]2(PO4)4 and K2.35Cu0.825[Cu3O]2(PO4)4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Single-Crystal X-ray Diffraction Study
3. Results
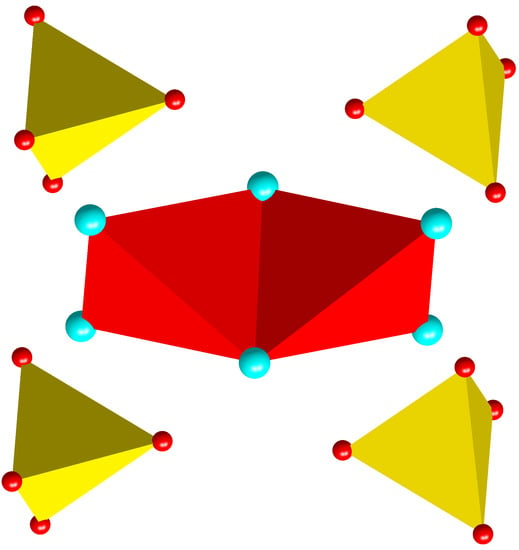
Crystal Structure Descriptions
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Atom | x | y | z | Ueq |
|---|---|---|---|---|
| Cu1 | 0.54313(10) | 0.21854(7) | 0.18319(7) | 0.00826(15) |
| Cu2 | 0.62938(11) | 0.54189(7) | 0.35677(7) | 0.01248(16) |
| Cu3 | 0.25788(9) | 0.51344(7) | 0.00581(7) | 0.00691(14) |
| Cu4 | 1 | 1 | 1 | 0.01075(19) |
| K1 | 1.1877(2) | −0.12671(17) | 0.39996(14) | 0.0251(3) |
| P1 | 0.5789(2) | −0.14031(14) | 0.18292(14) | 0.0066(2) |
| P2 | 0.9306(2) | 0.67348(14) | 0.72047(13) | 0.0053(2) |
| O1 | 0.7258(6) | −0.2413(4) | 0.3016(4) | 0.0146(7) |
| O2 | 0.5839(6) | 0.0261(4) | 0.2838(4) | 0.0102(7) |
| O3 | 0.3192(6) | −0.2209(4) | 0.1123(4) | 0.0134(7) |
| O4 | 0.6982(6) | −0.1156(4) | 0.0350(4) | 0.0106(7) |
| O5 | 0.9594(5) | 0.5576(4) | 0.8563(4) | 0.0093(6) |
| O6 | 1.1450(5) | 0.6696(4) | 0.6400(4) | 0.0074(6) |
| O7 | 0.6911(5) | 0.6036(4) | 0.5917(4) | 0.0093(6) |
| O8 | 0.9150(6) | 0.8492(4) | 0.7897(4) | 0.0120(7) |
| O9 | 0.5292(5) | 0.4366(4) | 0.1373(4) | 0.0066(6) |
| Atom | S.O.F. | x | y | z | Ueq |
|---|---|---|---|---|---|
| Cu1 | 1 | 0.84435(4) | 0.42009(6) | 0.88948(4) | 0.01789(15) |
| Cu2 | 1 | 0.92922(4) | 0.41660(6) | 0.73252(4) | 0.01725(15) |
| Cu3 | 1 | 0.75839(4) | 0.54529(5) | 0.73128(4) | 0.01226(14) |
| Cu4 | 1 | 0.74882(4) | 0.30406(5) | 0.75677(4) | 0.01233(14) |
| Cu5 | 1 | 0.57404(4) | 0.39718(6) | 0.75159(4) | 0.01062(13) |
| Cu6 | 1 | 0.43001(4) | 0.59447(7) | 0.74471(5) | 0.02626(19) |
| Cu7 | 1 | 0.33900(4) | 0.56147(6) | 0.89307(4) | 0.01664(15) |
| Cu8 | 1 | 0.25552(4) | 0.44950(5) | 0.73520(4) | 0.01493(15) |
| Cu9 | 1 | 0.24989(4) | 0.69237(5) | 0.76032(4) | 0.01117(14) |
| Cu10 | 1 | 0.07531(4) | 0.59072(5) | 0.75522(4) | 0.01117(13) |
| Cu11 | 1 | 0.16550(4) | 0.58938(6) | 0.60214(4) | 0.01817(15) |
| Cu12 | 1 | 0.25872(5) | 0.82651(7) | 0.49436(5) | 0.0335(2) |
| Cu13 | 1 | 0.34155(4) | 0.58442(6) | 0.39884(4) | 0.01298(14) |
| Cu14 | 0.5 | 0.10290(9) | 0.86289(12) | 0.34216(9) | 0.0188(3) |
| Cu15 | 0.15 | 0.0595(3) | 0.8514(4) | 0.3650(3) | 0.0188(3) |
| K1 | 1 | 0.23938(9) | 0.32812(12) | 0.49506(9) | 0.0313(3) |
| K2A | 0.41 | 0.4229(4) | 0.8501(4) | 0.6448(3) | 0.0718(19) |
| K2B | 0.59 | 0.4599(2) | 0.8680(3) | 0.5471(3) | 0.0613(11) |
| K3A | 0.42 | 0.4539(10) | 0.335(3) | 0.5443(9) | 0.078(6) |
| K3B | 0.36 | 0.4585(5) | 0.3883(10) | 0.5623(9) | 0.0382(19) |
| K3C | 0.22 | 0.445(2) | 0.346(4) | 0.569(2) | 0.069(9) |
| K4A | 0.45 | 0.5346(3) | 0.6385(4) | 0.9684(4) | 0.0725(17) |
| K4B | 0.55 | 0.4942(3) | 0.7322(5) | 1.0182(2) | 0.0804(19) |
| K4C | 0.15 | 0.4597(14) | 0.8276(19) | 1.042(2) | 0.125(12) |
| K4D | 0.55 | 0.4772(3) | 0.9217(6) | 1.0477(3) | 0.0828(17) |
| P1 | 1 | 0.90143(8) | 0.16223(11) | 0.83315(8) | 0.0099(3) |
| P2 | 1 | 0.90101(7) | 0.65707(11) | 0.82032(8) | 0.0080(2) |
| P3 | 1 | 0.40314(7) | 0.33475(11) | 0.81544(8) | 0.0090(2) |
| P4 | 1 | 0.40152(8) | 0.83626(11) | 0.83808(8) | 0.0111(3) |
| P5 | 1 | 0.34316(8) | 0.61861(13) | 0.57888(8) | 0.0144(3) |
| P6A | 0.337(6) | 0.3350(19) | 1.0893(18) | 0.5736(18) | 0.011(3) |
| P6B | 0.663(6) | 0.3349(9) | 1.0612(9) | 0.5739(10) | 0.0129(17) |
| P7 | 1 | 0.16036(8) | 1.05119(12) | 0.42170(8) | 0.0130(3) |
| P8 | 1 | 0.16418(8) | 0.62317(12) | 0.41864(8) | 0.0125(3) |
| O1 | 1 | 0.9538(2) | 0.1157(4) | 0.7643(3) | 0.0240(10) |
| O2 | 1 | 0.8989(3) | 0.0726(4) | 0.9004(3) | 0.0289(11) |
| O3 | 1 | 0.9302(2) | 0.2838(3) | 0.8587(2) | 0.0173(8) |
| O4 | 1 | 0.8155(2) | 0.1732(3) | 0.7957(2) | 0.0161(8) |
| O5 | 1 | 0.9596(2) | 0.5784(3) | 0.7737(2) | 0.0141(8) |
| O6 | 1 | 0.8775(3) | 0.5963(3) | 0.8971(2) | 0.0192(9) |
| O7 | 1 | 0.8277(2) | 0.6751(3) | 0.7635(2) | 0.0152(8) |
| O8 | 1 | 0.9385(2) | 0.7797(3) | 0.8364(2) | 0.0105(7) |
| O9 | 1 | 0.4379(2) | 0.2116(3) | 0.8333(2) | 0.0162(8) |
| O10 | 1 | 0.3249(2) | 0.3197(3) | 0.7645(2) | 0.0167(8) |
| O11 | 1 | 0.4600(2) | 0.4076(3) | 0.7625(2) | 0.0165(8) |
| O12 | 1 | 0.3874(3) | 0.3991(3) | 0.8928(2) | 0.0194(8) |
| O13 | 1 | 0.4321(2) | 0.7117(3) | 0.8570(3) | 0.0210(9) |
| O14 | 1 | 0.4578(2) | 0.9000(4) | 0.7807(3) | 0.0239(10) |
| O15 | 1 | 0.3901(3) | 0.9127(4) | 0.9113(3) | 0.0320(11) |
| O16 | 1 | 0.3197(2) | 0.8238(3) | 0.7932(2) | 0.0159(8) |
| O17 | 1 | 0.3869(2) | 0.6187(4) | 0.5003(2) | 0.0272(10) |
| O18A | 0.337(6) | 0.3132(7) | 0.7541(10) | 0.5773(7) | 0.0199(12) |
| O18B | 0.663(6) | 0.2644(4) | 0.6911(5) | 0.5798(4) | 0.0199(12) |
| O19A | 0.337(6) | 0.4069(9) | 0.6053(15) | 0.6410(9) | 0.021(3) |
| O19B | 0.663(6) | 0.4004(4) | 0.6670(8) | 0.6433(5) | 0.0221(17) |
| O20A | 0.337(6) | 0.2739(8) | 0.5379(11) | 0.5834(8) | 0.0258(13) |
| O20B | 0.663(6) | 0.3189(4) | 0.4881(6) | 0.5983(4) | 0.0258(13) |
| O21 | 1 | 0.4024(3) | 1.0565(5) | 0.6340(3) | 0.0390(13) |
| O22A | 0.337(6) | 0.2806(18) | 0.9835(18) | 0.5575(14) | 0.044(6) |
| O22B | 0.663(6) | 0.3011(7) | 0.9367(8) | 0.5635(7) | 0.030(2) |
| O23 | 1 | 0.2682(3) | 1.1526(5) | 0.6017(3) | 0.0374(12) |
| O24 | 1 | 0.3724(3) | 1.1141(5) | 0.4974(3) | 0.0325(11) |
| O25 | 1 | 0.1884(3) | 0.9209(4) | 0.4246(3) | 0.0269(10) |
| O26 | 1 | 0.2261(2) | 1.1350(4) | 0.3955(2) | 0.0189(8) |
| O27 | 1 | 0.0901(2) | 1.0434(4) | 0.3599(2) | 0.0271(10) |
| O28 | 1 | 0.1255(3) | 1.0924(4) | 0.5000(2) | 0.0209(9) |
| O29 | 1 | 0.1786(3) | 0.4971(3) | 0.3940(2) | 0.0228(9) |
| O30 | 1 | 0.1071(2) | 0.6862(4) | 0.3573(2) | 0.0199(9) |
| O31 | 1 | 0.2454(2) | 0.6921(3) | 0.4201(2) | 0.0147(8) |
| O32 | 1 | 0.1241(2) | 0.6348(4) | 0.5000(2) | 0.0191(8) |
| O33 | 1 | 0.8264(2) | 0.4268(3) | 0.7770(2) | 0.0094(7) |
| O34 | 1 | 0.6801(2) | 0.4241(3) | 0.7134(2) | 0.0092(7) |
| O35 | 1 | 0.3253(2) | 0.5669(3) | 0.7799(2) | 0.0110(7) |
| O36 | 1 | 0.1812(2) | 0.5730(3) | 0.7141(2) | 0.0116(7) |
References
- Han, T.-H.; Singleton, J.; Schlueter, J.A. Barlowite: A spin-1212 antiferromagnet with a geometrically perfect kagome motif. Phys. Rev. Lett. 2014, 113, 227203. [Google Scholar] [CrossRef] [Green Version]
- Norman, M.R. Herbertsmithite and the search for the quantum spin liquid. Rev. Mod. Phys. 2016, 88, 041002. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.G.; Kawae, T.; Kashitani, Y.; Li, C.S.; Tateiwa, N.; Takeda, K.; Yamada, H.; Xu, C.N.; Ren, Y. Unconventional magnetic transitions in the mineral clinoatacamite Cu2Cl(OH)3. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 052409. [Google Scholar] [CrossRef]
- Zheng, X.G.; Mori, T.; Nishiyama, K.; Higemoto, W.; Yamada, H.; Nishikubo, K.; Xu, C.N. Antiferromagnetic transitions in polymorphous minerals of the natural cuprates atacamite and botallackite Cu2Cl(OH)3. Phys. Rev. B Condens. Matter Mater. Phys. 2005, 71, 174404. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zhang, Q.-M. Structure and magnetism of S = 1/2 kagome antiferromagnets NiCu3(OH)6Cl2 and CoCu3(OH)6Cl2. J. Phys. Condens. Matter. 2013, 25, 026003. [Google Scholar] [CrossRef]
- Malcherek, T.; Mihailova, B.; Welch, M.D. Structural phase transitions of clinoatacamite and the dynamic Jahn-Teller effect. Phys. Chem. Miner. 2017, 44, 307–321. [Google Scholar] [CrossRef]
- Malcherek, T.; Welch, M.D.; Williams, P.A. The atacamite family of minerals—A testbed for quantum spin liquids. Acta Crystallogr. 2018, B74, 519–526. [Google Scholar] [CrossRef]
- Vilminot, S.; André, G.; Richard-Plouet, M.; Bourée-Vigneron, F.; Kurmoo, M. Magnetic Structure and Magnetic Properties of Synthetic Lindgrenite, Cu3(OH)2(MoO4)2. Inorg. Chem. 2006, 45, 10938–10946. [Google Scholar] [CrossRef] [PubMed]
- Belik, A.A.; Koo, H.-J.; Whangbo, M.-H.; Tsujii, N.; Naumov, P.; Takayama-Muromachi, E. Magnetic Properties of Synthetic Libethenite Cu2PO4OH: A New Spin-Gap System. Inorg. Chem. 2007, 46, 8684–8689. [Google Scholar] [CrossRef] [PubMed]
- Janson, O.; Tsirlin, A.A.; Schmitt, M.; Rosner, H. Large quantum fluctuations in the strongly coupled spin-1/2 chains of green dioptase Cu6Si6O18∙6H2O. Phys. Rev. B Condens. Matter Mater. Phys. 2010, 82, 014424. [Google Scholar] [CrossRef] [Green Version]
- Podlesnyak, A.; Prokhorenko, O.; Nikitin, S.E.; Kolesnikov, A.I.; Matsuda, M.; Dissanayake, S.E.; Prisk, T.R.; Nojiri, H.; Díaz-Ortega, I.F.; Kidder, M.K.; et al. magnetic ground state and magnetic excitations in black dioptase Cu6Si6O18. Phys. Rev. B Condens. Matter Mater. Phys. 2019, 100, 184401. [Google Scholar] [CrossRef]
- Hiroi, Z.; Ishikawa, H.; Yoshida, H.; Yamaura, J.-I.; Okamoto, Y. Orbital Transitions and Frustrated Magnetism in the Kagome-Type Copper Mineral Volborthite. Inorg. Chem. 2019, 58, 11949–11960. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Michiue, Y.; Takayama-Muromachi, E.; Isobe, M. β-Vesignieite BaCu3V2O8(OH)2: A structurally perfect S = 1/2 kagomé antiferromaget. J. Matter. Chem. 2012, 22, 18793–18796. [Google Scholar] [CrossRef]
- Boldrin, D.; Knight, K.; Wills, A.S. Orbital frustration in the S = 1/2 kagome magnet vesignieite, BaCu3V2O8(OH)2. J. Matter. Chem. C 2016, 4, 10315–10322. [Google Scholar] [CrossRef]
- Sun, W.; Huang, Y.-X.; Pan, Y.; Mi, J.-X. Synthesis and magnetic properties of centennialite: A new S = 1/2 Kagomé antiferromagnet and comparison with herbertsmithite and kapellasite. Phys. Chem. Miner. 2016, 43, 127–136. [Google Scholar] [CrossRef]
- Vilminot, S.; Richard-Plouet, M.; André, G.; Swierczynski, D.; Guillot, M.; Bourée-Vigneron, F.; Drillon, M.; André, G.; Swierczynski, D.; Guillot, M.; et al. Magnetic structure and properties of Cu3(OH)4SO4 made of triple chains of spins s = 1/2. J. Solid State Chem. 2003, 170, 255–264. [Google Scholar] [CrossRef]
- Brandão, P.; Rocha, J.; Reis, M.S.; dos Santos, A.M.; Jin, R. Magnetic properties of KNaMSi4O10 compounds (M = Mn, Fe, Cu). J. Solid State Chem. 2009, 182, 253–258. [Google Scholar] [CrossRef]
- Jahn, H.A.; Teller, E. Stability of polyatomic molecules in degenerate electronic states. Proc. R. Soc. Ser. A 1937, 161, 220–235. [Google Scholar]
- Hathaway, B.J. A new look at the stereochemistry and electronic properties of complexes of the copper(II) ion. In Complex Chemistry. Structure and Bonding; Emsley, J., Ernst, R.D., Hathaway, B.J., Warren, K.D., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; Volume 57, pp. 55–118. [Google Scholar]
- Burns, P.C.; Hawthorne, F.C. Static and dynamic Jahn-Teller effects in Cu2+ oxysalt minerals. Can. Mineral. 1996, 34, 1089–1105. [Google Scholar]
- Effenberger, H. Contribution to the Stereochemistry of Copper. The Transition from a Tetragonal Pyramidal to a Trigonal Bipyramidal Cu(II)O5 Coordination Figure with a Structure Determination of PbCu2(SeO3)3. J. Solid State Chem. 1988, 73, 118–126. [Google Scholar] [CrossRef]
- Eby, R.K.; Hawthorne, F.C. Structural Relations in Copper Oxysalt Minerals. I. Structural Hierarchy. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 1993, 49, 28–56. [Google Scholar] [CrossRef]
- Burns, P.C.; Hawthorne, F.C. Coordination-geometry structural pathways in Cu2+ oxysalt minerals. Can. Mineral. 1995, 33, 889–905. [Google Scholar]
- Krivovichev, S.V.; Filatov, S.K. Structural principles for minerals and inorganic compounds containing anion-centered tetrahedra. Am. Mineral. 1999, 84, 1099–1106. [Google Scholar] [CrossRef]
- Krivivochev, S.V.; Mentré, O.; Siidra, O.I.; Colmont, M.; Filatov, S.K. Anion-Centered Tetrahedra in Inorganic Compounds. Chem. Rev. 2013, 113, 6459–6535. [Google Scholar] [CrossRef]
- Volkova, L.M.; Marinin, D.V. Frustrated Antiferromagnetic Spin Chains of Edge-Sharing Tetrahedra in Volcanic Minerals K3Cu3(Fe0.82Al0.18)O2(SO4)4 and K4Cu4O2(SO4)4MeCl. J. Supercond. Nov. Magn. 2017, 30, 959–971. [Google Scholar] [CrossRef] [Green Version]
- Badrtdinov, D.I.; Kuznetsova, E.S.; Verchenko, V.Y.; Berdonosov, P.S.; Dolgikh, V.A.; Mazurenko, V.V.; Tsirlin, A.A. magnetism of coupled spin tetrahedra in ilinskite-type KCu5O2(SeO3)2Cl3. Sci. Rep. 2018, 8, 2379. [Google Scholar] [CrossRef] [Green Version]
- Botana, A.S.; Zheng, H.; Lapidus, S.H.; Mitchell, J.F.; Norman, M.R. Averievite: A copper oxide kagome antiferromagnet. Phys. Rev. B 2018, 98, 054421. [Google Scholar] [CrossRef] [Green Version]
- Winiarski, M.J.; Tran, T.T.; Chamorro, J.R.; McQueen, T.M. (CsX)Cu5O2(PO4)2 (X = Cl, Br, I): A Family of Cu2+ S = ½ Compounds with Capped-Kagomé Networks Composed of OCu4 Units. Inorg. Chem. 2019, 58, 4328–4336. [Google Scholar] [CrossRef]
- Siidra, O.I.; Vladimirova, V.A.; Tsirlin, A.A.; Chukanov, N.V.; Ugolkov, V.L. Cu9O2(VO4)4Cl2, the first copper oxychloride vanadate: Mineralogically inspired synthesis and magnetic behavior. Inorg. Chem. 2020, 59, 2136–2143. [Google Scholar] [CrossRef] [PubMed]
- Fujihala, M.; Morita, K.; Mole, R.; Mitsuda, S.; Tohyama, T.; Yano, S.-I.; Yu, D.; Sota, S.; Kuwai, T.; Koda, A.; et al. Gapless spin liquid in a square-kagome lattice antiferromagnet. Nat. Commun. 2020, 11, 3429. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Vladimirova, V.A.; Siidra, O.I.; Krivovichev, S.V. Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study. Molecules 2021, 26, 1833. [Google Scholar] [CrossRef]
- Aksenov, S.M.; Borovikova, E.Y.; Mironov, V.S.; Yamnova, N.A.; Volkov, A.S.; Ksenofontov, D.A.; Gurbanova, O.A.; Dimitrova, O.V.; Deyneko, D.V.; Zvereva, E.A.; et al. Rb2CaCu6(PO4)4O2, a novel oxophosphate, with a shchurovskyite-type topology: Synthesis, structure, magnetic properties and crystal chemistry of rubidium copper phosphate. Acta Crystallogr. 2019, B75, 903–913. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Belakovskiy, D.I.; Yapaskurt, V.O.; Vigasina, M.F.; Sidorov, E.G.; Pushcharovsky, D.Y. New arsenate minerals from the Arsenatnaya fumarole, Tolbachik volcano, Kamchatka, Russia. IV. Shchurovskyite, K2CaCu6O2(AsO4)4 and dmisokolovite, K3Cu5AlO2(AsO4)4. Mineral. Mag. 2015, 79, 1737–1753. [Google Scholar] [CrossRef]
- Kornyakov, I.V.; Krivovichev, S.V.; Gurzhiy, V.V. Oxocentered Units in Three Novel Rb-Containing Copper Compounds Prepared by CVT Reactions Method. Z. Anorg. Allg. Chem. 2018, 644, 77–81. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Siidra, O.I.; Colmont, M.; Mentré, O.; Krivovichev, S.V. Emulating exhalative chemistry: Synthesis and structural characterization of ilinskite, Na[Cu5O2](SeO3)2Cl3, and its K-analogue. Miner. Petrol. 2015, 109, 421–430. [Google Scholar] [CrossRef]
- Jing, B.; Peng, C.; Wang, Y.; Liu, Q.; Tong, S.; Zhang, Y.; Ge, M. Hygroscopic properties of potassium chloride and its internal mixtures with organic compounds relevant to biomass burning aerosol particles. Sci. Rep. 2015, 7, 43572. [Google Scholar] [CrossRef] [Green Version]
- Giamarelou, M.; Smith, M.; Papapanagiotou, E.; Martin, S.; Biskos, G. Hygroscopic properties of potassium-halide nanoparticles. Aerosol Sci. Technol. 2018, 52, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Brunel-Laügt, M.; Guitel, J.-C. Structure crystalline de Cu5O2(PO4)2. Acta Crystallogr. 1977, B33, 3465–3468. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, version 1.171.38.46; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Agakhanov, A.A.; Pushcharovsky, D.Y.; Yapaskurt, V.O.; Belakovskiy, D.I.; Vigasina, M.F.; Sidrov, E.G.; Britvin, S.N. Cryptochalcite, K2Cu5O(SO4)5, and cesiodymite, CsKCu5O(SO4)5, two new isotypic minerals and the K-Cs isomorphism in this solid-solution series. Eur. J. Mineral. 2018, 30, 593–607. [Google Scholar] [CrossRef]
- Siidra, O.I.; Lukina, E.A.; Nazarchuk, E.V.; Depmeier, W.; Bubnova, R.S.; Agakhanov, A.A.; Avdontseva, E.Y.; Filatov, S.K.; Kovrugin, V.M. Saranchinaite, Na2Cu(SO4)2, a new exhalative mineral from Tolbachik volcano, Kamchatka, Russia, and a product of the reversible dehydration of kröhnkite, Na2Cu(SO4)2(H2O)2. Mineral. Mag. 2018, 82, 257–274. [Google Scholar] [CrossRef]
- Berry, R.S. Correlation of Rates of Intramolecular Tunneling Processes, with Application to Some Group V Compounds. J. Chem. Phys. 1960, 32, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Pasquarello, A.; Petri, I.; Salmon, P.S.; Parisel, O.; Car, R.; Tóth, E.; Powell, D.H.; Fischer, H.E.; Helm, L.; Merbach, A.E. First Solvation Shell of the Cu(II) Aqua Ion: Evidence for Fivefold Coordination. Science 2001, 291, 856–859. [Google Scholar] [CrossRef]
- McCusker, L.B.; Liebau, F.; Engelhardt, G. Nomenclature of structural and compositional characteristics of ordered microporous and mesoporous materials with inorganic hosts: (IUPAC recommendations 2001). Microporous Mesoporous Mater. 2003, 58, 3–13. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Topological complexity of crystal structures: Quantitative approach. Acta Crystallogr. 2012, A68, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Miner. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity and configurational entropy of crystalline solids. Acta Crystallogr. 2016, B72, 274–276. [Google Scholar]
- Krivovichev, S.V. Ladders of information: What contributes to the structural complexity in inorganic crystal. Z. Kristallogr. 2018, 233, 155–161. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef] [PubMed]



| Compound | 1 | 2 |
|---|---|---|
| Formula | K2Cu[Cu3O]2(PO4)4 | K2.35Cu0.825[Cu3O]2(PO4)4 |
| Space Group | P-1 | P21/n |
| a, Å | 5.7787(3) | 16.7138(4) |
| b, Å | 8.2612(4) | 11.2973(3) |
| c, Å | 8.3717(4) | 16.8031(4) |
| α, ° | 95.813(4) | 90 |
| β, ° | 103.239(4) | 90.775(2) |
| γ, ° | 96.821(4) | 90 |
| V, Å3 | 382.85(3) | 3172.47(13) |
| μ, mm−1 | 10.601 | 10.137 |
| Z | 1 | 8 |
| Dcalc, g/cm3 | 4.055 | 3.925 |
| Color | Light green | Intense green |
| Total reflections | 4126 | 37428 |
| Unique reflections | 1759 | 7219 |
| Reflection with |Fo|≥4σF | 1491 | 5761 |
| Angle range 2θ, °, MoKα | 5.01 to 54.998 | 6.492 to 55 |
| Rint, Rσ | 0.0286, 0.0359 | 0.0368, 0.0303 |
| R1, wR2 (|Fo| ≥ 4σF) | 0.0285, 0.0602 | 0.0394, 0.0847 |
| R1, wR2 (all data) | 0.0368, 0.0634 | 0.0535, 0.0901 |
| GOOF | 1.078 | 1.032 |
| ρmin, ρmax, e/Å3 | 0.69 / −0.64 | 1.57 / −1.94 |
| CSD | 2092265 | 2092266 |
| Compound | K2Cu[Cu3O]2(PO4)4 | K2.35Cu0.825[Cu3O]2(PO4)4 | Rb2Ca[Cu3O]2(PO4)4 | K2Ca[Cu3O]2(AsO4)4 | K3[Cu5AlO2](AsO4)4 |
|---|---|---|---|---|---|
| Space Group | P-1 | P21/n | C2 | C2 | C2/c |
| a, Å | 5.779 | 16.714 | 16.891 | 17.286 | 17.085 |
| b, Å | 8.261 | 11.297 | 5.641 | 5.670 | 5.719 |
| c, Å | 8.372 | 16.803 | 8.359 | 8.573 | 16.533 |
| α, β, γ, ° | 95.81, 103.24, 96.82 | 90, 90.77, 90 | 90, 93.92, 90 | 90, 92.95, 90 | 90, 91.72, 90 |
| V, Å3 | 382.8 | 3172.5 | 794.6 | 839.2 | 1614.7 |
| Z | 1 | 8 | 2 | 2 | 4 |
| v, atoms/cell | 31 | 250 | 31 | 31 | 62 |
| IG, bits/atom | 3.986 | 5.974 * | 4.051 | 4.051 | 4.051 |
| IG, total, bits/cell | 123.58 | 1493.446 * | 125.58 | 125.58 | 251.16 |
| Reference | This work | This work | 33 | 34 | 34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornyakov, I.V.; Krivovichev, S.V. Crystal Chemical Relations in the Shchurovskyite Family: Synthesis and Crystal Structures of K2Cu[Cu3O]2(PO4)4 and K2.35Cu0.825[Cu3O]2(PO4)4. Crystals 2021, 11, 807. https://doi.org/10.3390/cryst11070807
Kornyakov IV, Krivovichev SV. Crystal Chemical Relations in the Shchurovskyite Family: Synthesis and Crystal Structures of K2Cu[Cu3O]2(PO4)4 and K2.35Cu0.825[Cu3O]2(PO4)4. Crystals. 2021; 11(7):807. https://doi.org/10.3390/cryst11070807
Chicago/Turabian StyleKornyakov, Ilya V., and Sergey V. Krivovichev. 2021. "Crystal Chemical Relations in the Shchurovskyite Family: Synthesis and Crystal Structures of K2Cu[Cu3O]2(PO4)4 and K2.35Cu0.825[Cu3O]2(PO4)4" Crystals 11, no. 7: 807. https://doi.org/10.3390/cryst11070807
APA StyleKornyakov, I. V., & Krivovichev, S. V. (2021). Crystal Chemical Relations in the Shchurovskyite Family: Synthesis and Crystal Structures of K2Cu[Cu3O]2(PO4)4 and K2.35Cu0.825[Cu3O]2(PO4)4. Crystals, 11(7), 807. https://doi.org/10.3390/cryst11070807