Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions
Abstract
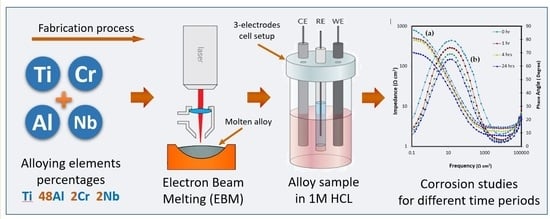
:1. Introduction
2. Materials and Methods
2.1. Electron Beam Melting of γ-TiAl
2.2. Electrochemical Experiments
2.3. Surface Characterization
3. Results and Discussion
3.1. Electrochemical Measurements
3.2. Electrochemical Impedance Spectroscopy (EIS)
3.3. Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray (EDX) Analyses
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, X. Review of alloy and process development of TiAl alloys. Intermetallics 2006, 14, 1114–1122. [Google Scholar] [CrossRef]
- Bewlay, B.P.; Nag, S.; Suzuki, A.; Weimer, M.J. TiAl alloys in commercial aircraft engines. Mater. High Temp. 2016, 33, 549–559. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.K.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R Rep. 2004, 47, 49–121. [Google Scholar] [CrossRef] [Green Version]
- Aspinwall, D.K.; Dewes, R.C.; Mantle, A.L. The Machining of γ-TiAI Intermetallic Alloys. CIRP Ann. Manuf. Technol. 2005, 54, 99–104. [Google Scholar] [CrossRef]
- Kim, Y.-W. Microstructural evolution and mechanical properties of a forged gamma titanium aluminide alloy. Acta Metall. Mater. 1992, 40, 1121–1134. [Google Scholar] [CrossRef]
- Schwaighofer, E.; Clemens, H.; Mayer, S.; Lindemann, J.; Klose, J.; Smarsly, W.; Güther, V. Microstructural design and mechanical properties of a cast and heat-treated intermetallic multi-phase γ-TiAl based alloy. Intermetallics 2014, 44, 128–140. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ceylan, A.; Martinez, E.; Martinez, J.L.; Hernandez, D.H.; Machado, B.I.; Ramirez, D.A.; Medina, F.; Collins, S.; et al. Characterization of titanium aluminide alloy components fabricated by additive manufacturing using electron beam melting. Acta Mater. 2010, 58, 1887–1894. [Google Scholar] [CrossRef]
- Delgado-Alvarado, C.; Sundaram, P.A. Corrosion evaluation of Ti-48Al-2Cr-2Nb (at. %) in Ringer’s solution. Acta Biomater. 2006, 2, 701–708. [Google Scholar] [CrossRef]
- Abdo, H.S.; Sherif, E.-S.M.; El-Serehy, H.A. Manufacturing of Ti-6%Al and Ti-6%Al-4%V Alloys and Their Corrosion in Sodium Chloride Solutions. Crystals 2020, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Abdo, H.S.; Seikh, A.H.; Mandal, B.B.; Mohammed, J.A.; Ragab, S.A.; Abdo, M.S. Microstructural Characterization and Corrosion-Resistance Behavior of Dual-Phase Steels Compared to Conventional Rebar. Crystals 2020, 10, 1068. [Google Scholar] [CrossRef]
- Luqman, M.; Seikh, A.H.; Sarkar, A.; Ragab, S.A.; Mohammed, J.A.; Ijaz, M.F.; Abdo, H.S. A Comparative Study of the Electrochemical Behavior of α and β Phase Ti6Al4V Alloy in Ringer’s Solution. Crystals 2020, 10, 190. [Google Scholar] [CrossRef] [Green Version]
- Seikh, A.H.; Baig, M.; Singh, J.K.; Mohammed, J.A.; Luqman, M.; Abdo, H.S.; Khan, A.R.; Alharthi, N.H. Microstructural and Corrosion Characteristics of Al-Fe Alloys Produced by High-Frequency Induction-Sintering Process. Coatings 2019, 9, 686. [Google Scholar] [CrossRef] [Green Version]
- De Angelis, N.; Solimei, L.; Pasquale, C.; Alvito, L.; Lagazzo, A.; Barberis, F. Mechanical Properties and Corrosion Resistance of TiAl6V4 Alloy Produced with SLM Technique and Used for Customized Mesh in Bone Augmentations. Appl. Sci. 2021, 11, 5622. [Google Scholar] [CrossRef]
- Zubaidy, E.A.H.A.; Mohammad, F.S.; Bassioni, G. Effect of pH, Salinity and Temperature on Aluminum Cookware Leaching during Food Preparation. Int. J. Electrochem. Sci. 2011, 6, 6424–6441. [Google Scholar]
- Darowicki, K.; Krakowiak, S.; Ślepski, P. Evaluation of pitting corrosion by means of dynamic electrochemical impedance spectroscopy. Electrochim. Acta 2004, 49, 2909–2918. [Google Scholar] [CrossRef]
- Floyd, F.L.; Avudaiappan, S.; Gibson, J.; Mehta, B.; Smith, P.; Provder, T.; Escarsega, J. Using electrochemical impedance spectroscopy to predict the corrosion resistance of unexposed coated metal panels. Prog. Org. Coat. 2009, 66, 8–34. [Google Scholar] [CrossRef]
- Abdo, H.S.; Seikh, A.H.; Mohammed, J.A.; Luqman, M.; Ragab, S.A.; Almotairy, S.M. Influence of Chloride Ions on Electrochemical Corrosion Behavior of Dual-Phase Steel over Conventional Rebar in Pore Solution. Appl. Sci. 2020, 10, 4568. [Google Scholar] [CrossRef]
- Cimpoeșu, R.; Vizureanu, P.; Știrbu, I.; Sodor, A.; Zegan, G.; Prelipceanu, M.; Cimpoeșu, N.; Ioanid, N. Corrosion-Resistance Analysis of HA Layer Deposited through Electrophoresis on Ti4Al4Zr Metallic Substrate. Appl. Sci. 2021, 11, 4198. [Google Scholar] [CrossRef]
- Shinde, V.; Patil, P.P. Evaluation of corrosion protection performance of poly (o-ethyl aniline) coated copper by electrochemical impedance spectroscopy. Mater. Sci. Eng. B 2010, 168, 142–150. [Google Scholar] [CrossRef]
- Gao, W.; Cao, S.; Yang, Y.; Wang, H.; Li, J.; Jiang, Y. Electrochemical impedance spectroscopy investigation on indium tin oxide films under cathodic polarization in NaOH solution. Thin Solid Films 2012, 520, 6916–6921. [Google Scholar] [CrossRef]
- Hassan, A.H.H.E.; Abdelghani, M.A. Amin. Electrochim. Acta 2007, 52, 6359. [Google Scholar] [CrossRef]
- Bommersbach, B.P.C.; Alemany-Dumont, J.P.; Millet, B. Normand. Electrochim. Acta 2005, 51, 1076. [Google Scholar] [CrossRef]
- Johnson, W.B.; Macdonald, J.R. Theory in Impedance Spectroscopy, Experiments and Applications; John Wiley & Sons: New York, NY, USA, 2005. [Google Scholar]












| Parameter | Level |
|---|---|
| Voltage | 60 KV |
| Beam current | 25 mA |
| Beam speed | 2400 mm/s |
| Layer thickness | 90 microns |
| Nominal Build temperature | 1100 °C |
| Controlled vacuum | ~2 × 10−3 mBar |
| Exposure Period/h | Parameter | ||||
|---|---|---|---|---|---|
| βa/mV·dec−1 | βc/mV·dec−1 | ECorr/mV | jCorr/µA·cm−2 | Rp/kΩ·cm2 | |
| 0 | 12.76 | 15.72 | −674 | 0.857 | 3.568 |
| 1 | 13.69 | 15.51 | −653 | 0.955 | 3.306 |
| 4 | 14.91 | 17.15 | −634 | 1.456 | 2.378 |
| 24 | 15.62 | 17.34 | −604 | 2.005 | 1.779 |
| Temperature/°C | Parameter | ||||
|---|---|---|---|---|---|
| βa/mV·dec−1 | βc/mV·dec−1 | ECorr/mV | jCorr/µA·cm−2 | Rp/kΩ·cm2 | |
| 30 | 13.17 | 16.02 | −603 | 0.926 | 3.389 |
| 40 | 13.46 | 16.17 | −611 | 0.985 | 3.238 |
| 50 | 15.74 | 17.22 | −625 | 1.364 | 2.618 |
| 60 | 16.48 | 17.94 | −638 | 1.925 | 1.937 |
| Exposure Period/h | RS/Ω·cm2 | Q | Rct/Ω·cm2 | |
|---|---|---|---|---|
| CPE (mMho) | n | |||
| 0 | 3.22 | 0.534 | 0.946 | 880 |
| 1 | 3.83 | 0.712 | 0.992 | 520 |
| 4 | 3.19 | 0.901 | 0.892 | 460 |
| 24 | 3.43 | 1.294 | 0.821 | 230 |
| Temperature/°C | RS/Ω·cm2 | Q | Rct/Ω·cm2 | |
|---|---|---|---|---|
| CPE (mMho) | n | |||
| 30 | 3.48 | 0.594 | 0.918 | 764 |
| 40 | 3.69 | 0.662 | 0.903 | 676 |
| 50 | 3.42 | 0.786 | 0.885 | 512 |
| 60 | 3.35 | 0.998 | 0.847 | 446 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdo, H.S.; Abdus Samad, U.; Mohammed, J.A.; Ragab, S.A.; Seikh, A.H. Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions. Crystals 2021, 11, 889. https://doi.org/10.3390/cryst11080889
Abdo HS, Abdus Samad U, Mohammed JA, Ragab SA, Seikh AH. Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions. Crystals. 2021; 11(8):889. https://doi.org/10.3390/cryst11080889
Chicago/Turabian StyleAbdo, Hany S., Ubair Abdus Samad, Jabair Ali Mohammed, Sameh A. Ragab, and Asiful H. Seikh. 2021. "Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions" Crystals 11, no. 8: 889. https://doi.org/10.3390/cryst11080889
APA StyleAbdo, H. S., Abdus Samad, U., Mohammed, J. A., Ragab, S. A., & Seikh, A. H. (2021). Mitigating Corrosion Effects of Ti-48Al-2Cr-2Nb Alloy Fabricated via Electron Beam Melting (EBM) Technique by Regulating the Immersion Conditions. Crystals, 11(8), 889. https://doi.org/10.3390/cryst11080889