Synthesis of Novel Aqua ƞ4-NNNO/Cu(II) Complexes as Rapid and Selective Oxidative Catalysts for O-Catechol: Fluorescence, Spectral, Chromotropism and Thermal Analyses
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Measurements
2.2. Synthesis
2.2.1. Synthesis of SB Ligand
2.2.2. Synthesis of Cu(II) Complexes: General Procedure
Complex 1
Complex 2
Complex 3
2.3. Catalytic Oxidation of Catechol
3. Results and Discussion
3.1. Synthesis and Characterization
3.2. Electronic Absorption and Fluorescence Spectra
3.3. Stoichiometric Titration
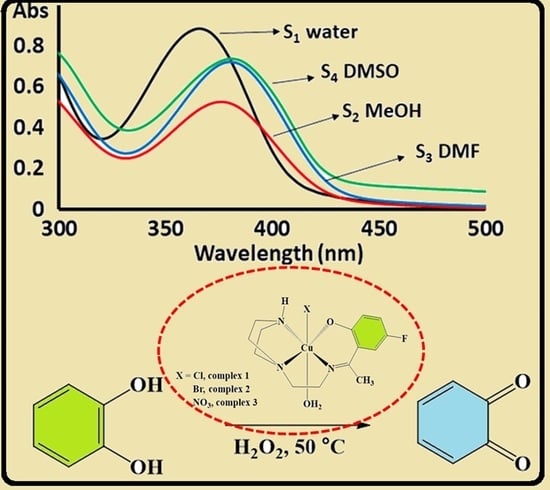
3.4. Halochromism and Solvatochromism
3.5. Thermogravimetric Analysis
3.6. Electrolytic Conductivity
3.7. Catalytic Oxidation of Catechol
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pearson, R.G. Chemical Hardness; Wiley-VCH Verlag GmbH: Weinheim, Germany, 1997; pp. 197–198. [Google Scholar] [CrossRef]
- Duncan, C.; White, A.R. Copper complexes as therapeutic agents. Metallomics 2012, 4, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Santiago, P.H.O.; Aiube, C.M.; de Macedo, J.L.; Gatto, C.C. Hydrazone-derived copper(II) coordination polymer as a selective liquid-phase catalyst: Synthesis, crystal structure and performance towards benzyl alcohol oxidation. Mol. Catal. 2020, 496, 111177. [Google Scholar] [CrossRef]
- Schiff, H. Mittheilungen aus dem Universitätslaboratorium in Pisa: Eine neue Reihe organischer Basen. Justus Liebigs Ann. Chem. 1864, 131, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Gupta, K.C.; Sutar, A.K. Catalytic activities of Schiff base transition metal complexes. Coord. Chem. Rev. 2008, 252, 1420–1450. [Google Scholar] [CrossRef]
- Canali, L.; Sherrington, D.C. Utilisation of homogeneous and supported chiral metal(salen) complexes in asymmetric catalysis. Chem. Soc. Rev. 1999, 28, 85–93. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Al-Hamdani, A.A.S.; Kaseem, M. Synthesis and antioxidant activities of Schiff bases and their complexes: A review. Appl. Organometal. Chem. 2016, 30, 810–817. [Google Scholar] [CrossRef]
- More, M.S.; Joshi, P.G.; Mishra, Y.K.; Khanna, P.K. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Lacroix, P.G. Second-Order Optical Nonlinearities in Coordination Chemistry: The Case of Bis(salicylaldiminato)metal Schiff Base Complexes. Eur. J. Inorg. Chem. 2001, 2001, 339–348. [Google Scholar] [CrossRef]
- Badran, I.; Abdallah, L.; Mubarakeh, R.; Warad, I. Effect of alkyl derivation on the chemical and antibacterial properties of newly synthesized Cu(II)-diamine complexes. Mor. J. Chem. 2019, 7, 161–170. [Google Scholar]
- Punniyamurthy, T.; Rout, L. Recent advances in copper-catalyzed oxidation of organic compounds. Coord. Chem. Rev. 2008, 252, 134–154. [Google Scholar] [CrossRef]
- Gupta, K.C.; Kumar Sutar, A.; Lin, C.-C. Polymer-supported Schiff base complexes in oxidation reactions. Coord. Chem. Rev. 2009, 253, 1926–1946. [Google Scholar] [CrossRef]
- Fukuda, Y. Inorganic Chromotropism: Basic Concepts and Applications of Colored Materials; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Golchoubian, H.; Moayyedi, G.; Reisi, N. Halochromism, ionochromism, solvatochromism and density functional study of a synthesized copper(II) complex containing hemilabile amide derivative ligand. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 138, 913–924. [Google Scholar] [CrossRef]
- Kumari, S.; Mahato, A.K.; Maurya, A.; Singh, V.K.; Keshwarwani, N.; Kachhap, P.; Koshevoy, I.O.; Haldar, C. Syntheses and characterization of monobasic tridentate Cu(II) Schiff-base complexes for efficient oxidation of 3,5-di-tert-butylcatechol and oxidative bromination of organic substrates. New J. Chem. 2017, 41, 13625. [Google Scholar] [CrossRef]
- Maity, T.; Saha, D.; Bhunia, S.; Brandão, P.; Das, S.; Koner, S. A family of ligand and anion dependent structurally diverse Cu(II) Schiff–base complexes and their catalytic efficacy in O–arylation reaction in ethanolic medium. RSC Adv. 2015, 5, 8217. [Google Scholar] [CrossRef]
- Salih, K.S.M.; Shraim, A.M.; Al-Mhini, S.R.; Al-Soufi, R.E.; Warad, I. New tetradentate Schiff base Cu(II) complexes: Synthesis, physicochemical, chromotropism, fluorescence, thermal, and selective catalytic oxidation. Emergent Mater. 2021, 4, 423. [Google Scholar] [CrossRef]
- Uzun, S.; Demircioğlu, Z.; Taşdoğan, M.; Ağar, E. Quantum chemical and X-ray diffraction studies of (E)-3-(((3,4-dimethoxybenzyl)imino)methyl)benzene-1,2-diol. J. Mol. Struct. 2020, 1206, 127749. [Google Scholar] [CrossRef]
- Ziółek, M.; Kubicki, J.; Maciejewski, A.; Naskręcki, R.; Grabowska, A. An ultrafast excited state intramolecular proton transfer (ESPIT) and photochromism of salicylideneaniline (SA) and its “double” analogue salicylaldehyde azine (SAA). A controversial case. Phys. Chem. Chem. Phys. 2004, 6, 4682–4689. [Google Scholar] [CrossRef]
- Jankowska, J.; Rode, M.F.; Sadlej, J.; Sobolewski, A.L. Excited-state intramolecular proton transfer: Photoswitching in salicylidene methylamine derivatives. ChemPhysChem 2014, 15, 1643–1652. [Google Scholar] [CrossRef]
- Mathammal, R.; Sangeetha, K.; Sangeetha, M.; Mekala, R.; Gadheeja, S. Molecular structure, vibrational, UV, NMR, HOMO-LUMO, MEP, NLO, NBO analysis of 3,5 di tert butyl 4 hydroxy benzoic acid. J. Mol. Struct. 2016, 1120, 1–14. [Google Scholar] [CrossRef]
- Said, M.A.; Qasem, H.A.; Alzahrani, S.O.; Zarrouk, A.; Warad, I. Synthesis and XRD of neutral NiL complex using unsymmetrical ONNO tetradentate schiff base: Hirschfeld, spectral, DFT and thermal analysis. J. Coord. Chem. 2020, 73, 1280–1291. [Google Scholar] [CrossRef]
- Al-Zaqri, N.; Salih, K.S.M.; Awwadi, F.F.; Alsalme, A.; Alharthi, F.A.; Alsyahi, A.; Al Ali, A.; Zarrouk, A.; Aljohani, M.; Chetouni, A.; et al. Synthesis, physicochemical, thermal, and XRD/HSA interactions of mixed [Cu(Bipy)(Dipn)](X)2 complexes: DNA binding and molecular docking evaluation. J. Coord. Chem. 2020, 73, 3236–3248. [Google Scholar] [CrossRef]
- Hema, M.K.; Karthik, C.S.; Lokanath, N.K.; Mallu, P.; Zarrouk, A.; Salih, K.S.M.; Warad, I. Synthesis of novel Cubane [Ni4(O∩O)4(OCH3)4(OOH)4] cluster: XRD/HSA-interactions, spectral, DNA-binding, docking and subsequent thermolysis to NiO nanocrystals. J. Mol. Liq. 2020, 315, 113756. [Google Scholar] [CrossRef]
- Warad, I.; Musameh, S.; Badran, I.; Nassar, N.N.; Brandao, P.; Tavares, C.J.; Barakat, A. Synthesis, solvatochromism and crystal structure of trans-[Cu(Et2NCH2CH2NH2)2.H2O](NO3)2 complex: Experimental with DFT combination. J. Mol. Struct. 2017, 1148, 328–338. [Google Scholar] [CrossRef] [Green Version]
- Warad, I.; Awwadi, F.F.; Abd Al-Ghani, B.; Sawafta, A.; Shivalingegowda, N.; Lokanath, N.K.; Mubarak, M.S.; Ben Hadda, T.; Zarrouk, A.; Al-Rimawi, F.; et al. Ultrasound-assisted synthesis of two novel [CuBr(diamine)2.H2O]Br complexes: Solvatochromism, crystal structure, physicochemical, Hirshfeld surface thermal, DNA/binding, antitumor and antibacterial activities. Ultrason. Sonochem. 2018, 48, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mayer, U.; Gutmann, V.; Gerger, W. The acceptor number ? A quantitative empirical parameter for the electrophilic properties of solvents. Monatsh. Chem. 1975, 106, 1235–1257. [Google Scholar] [CrossRef]
- Kettle, S.F.A. Physical Inorganic Chemistry; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Singh, Y.; Patel, R.N.; Patel, S.K.; Patel, A.K.; Patel, N.; Singh, R.; Butcher, R.J.; Jasinski, J.P.; Gutierrez, A. Experimental and quantum computational study of two new bridgedcopper(II) coordination complexes as possible models for antioxidantsuperoxide dismutase: Molecular structures, X-band electronparamagnetic spectra and cryogenic magnetic properties. Polyhedron 2019, 171, 155. [Google Scholar] [CrossRef]
- Jakubowska, M.A.; Pyka, J.; Michalczyk-Wetula, D.; Baczynski, K.; Ciesla, M.; Susz, A.; Ferdek, P.; Płonka, B.K.; Fiedor, L.; Płonka, P.M. Electron paramagnetic resonance spectroscopy reveals alterations in the redox state of endogenous copper and iron complexes in photodynamic stress-induced ischemic mouse liver. Redox Biol. 2020, 34, 101566. [Google Scholar] [CrossRef]
- Wenker, E.; Siegler, M.A.; Lutz, M.; Bouwman, E. Catalytic catechol oxidation by copper complexes: Development of a structure-activity relationship. Dalton Trans. 2015, 44, 12196. [Google Scholar] [CrossRef] [Green Version]
- Mallat, T.; Baiker, A. Oxidation of alcohols with molecular oxygen on platinum metal catalysts in aqueous solutions. Catal. Today 1994, 19, 247. [Google Scholar] [CrossRef]
- Musawir, M.; Davey, P.N.; Kelly, G.; Kozhevnikov, I.V. Highly efficient liquid-phase oxidation of primary alcohols to aldehydes with oxygen catalysed by Ru-Co oxide. Chem. Commun. 2003, 12, 1414–1415. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.S.; Martins, L. Recent Advances in Copper Catalyzed Alcohol Oxidation in Homogeneous Medium. Molecules 2020, 25, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshaheri, A.A.; Tahir, M.I.M.; Abdul Rahman, M.B.; Begum, T.; Saleh, T.A. Synthesis, characterisation and catalytic activity of dithiocarbazate Schiff base complexes in oxidation of cyclohexane. J. Mol. Liq. 2017, 240, 486–496. [Google Scholar] [CrossRef]
- Mohamad, A.D.M.; El-Shrkawy, E.R.; Al-Hussein, M.F.I.; Adam, M.S.S. Water-soluble Cu(II)-complexes of Schiff base amino acid derivatives as biological reagents and sufficient catalysts for oxidation reactions. J. Taiwan Inst. Chem. Eng. 2020, 113, 27–45. [Google Scholar] [CrossRef]
- Bocian, A.; Gorczyński, A.; Marcinkowski, D.; Witomska, S.; Kubicki, M.; Mech, P.; Bogunia, M.; Brzeski, J.; Makowski, M.; Pawluć, P.; et al. New benzothiazole based copper(II) hydrazone Schiff base complexes for selective and environmentally friendly oxidation of benzylic alcohols: The importance of the bimetallic species tuned by the choice of the counter ion. J. Mol. Liq. 2020, 302, 112590. [Google Scholar] [CrossRef]













| Entry | Complex | Solvent | Conversion % a | Time (min) | TOF g |
|---|---|---|---|---|---|
| 1 | 1 | Water | >99 | 10 | 625 |
| 2 | 2 | Water | >99 | 15 | 400 |
| 3 | 3 | Water | >99 | 20 | 303 |
| 4 | 1 | DMSO | >99 | 15 | 400 |
| 5 | 1 | DMF | >99 | 18 | 333 |
| 6 | 1b | Water | >99 | 5 | 1250 |
| 7 | 1c | Water | >99 | 2 | 3333 |
| 8 | 1b, d | Water | 2 | 100 | 1 |
| 9 | 1b, e | Water | 10 | 100 | 5 |
| 10 | 1b, f | Water | 0 | 100 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shraim, A.M.; Salih, K.S.M.; Al-Soufi, R.E.; Al-Mhini, S.R.; Ahmad, M.I.; Warad, I. Synthesis of Novel Aqua ƞ4-NNNO/Cu(II) Complexes as Rapid and Selective Oxidative Catalysts for O-Catechol: Fluorescence, Spectral, Chromotropism and Thermal Analyses. Crystals 2021, 11, 1072. https://doi.org/10.3390/cryst11091072
Shraim AM, Salih KSM, Al-Soufi RE, Al-Mhini SR, Ahmad MI, Warad I. Synthesis of Novel Aqua ƞ4-NNNO/Cu(II) Complexes as Rapid and Selective Oxidative Catalysts for O-Catechol: Fluorescence, Spectral, Chromotropism and Thermal Analyses. Crystals. 2021; 11(9):1072. https://doi.org/10.3390/cryst11091072
Chicago/Turabian StyleShraim, Amjad M., Kifah S. M. Salih, Ranim E. Al-Soufi, Soaad R. Al-Mhini, Mohammad I. Ahmad, and Ismail Warad. 2021. "Synthesis of Novel Aqua ƞ4-NNNO/Cu(II) Complexes as Rapid and Selective Oxidative Catalysts for O-Catechol: Fluorescence, Spectral, Chromotropism and Thermal Analyses" Crystals 11, no. 9: 1072. https://doi.org/10.3390/cryst11091072
APA StyleShraim, A. M., Salih, K. S. M., Al-Soufi, R. E., Al-Mhini, S. R., Ahmad, M. I., & Warad, I. (2021). Synthesis of Novel Aqua ƞ4-NNNO/Cu(II) Complexes as Rapid and Selective Oxidative Catalysts for O-Catechol: Fluorescence, Spectral, Chromotropism and Thermal Analyses. Crystals, 11(9), 1072. https://doi.org/10.3390/cryst11091072