Understanding of Photophysical Processes in DIO Additive-Treated PTB7:PC71BM Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Fabrication of PSCs
2.2. Structure and Steady-state Spectra Measurements
2.3. TA and Time-Resolved Photoluminescence (TRPL) Measurements
3. Results and Discussion
3.1. Steady-State Absorption Properties
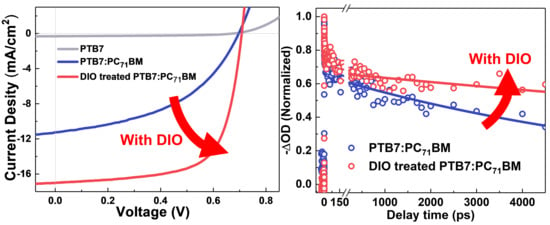
3.2. Morphology and Photovoltaic Properties
3.3. Electric Field Induced Exciton Dissociation
3.4. Charge Photogeneration and Recombination Processes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hou, J.; Inganäs, O.; Friend, R.H.; Gao, F. Organic solar cells based on non-fullerene acceptors. Nat. Mater. 2018, 17, 119–128. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, J.; Deng, W.; Luo, M.; Xie, Y.; Liang, Q.; Zou, Y.; He, Z.; Wu, H.; Cao, Y. High-efficiency organic solar cells with low non-radiative recombination loss and low energetic disorder. Nat. Photonics 2020, 14, 300–305. [Google Scholar] [CrossRef]
- Qi, F.; Jiang, K.; Lin, F.; Wu, Z.; Zhang, H.; Gao, W.; Li, Y.; Cai, Z.; Woo, H.Y.; Zhu, Z.; et al. Over 17% Efficiency binary organic solar cells with photoresponses reaching 1000 nm enabled by selenophene-fused nonfullerene acceptors. ACS Energy Lett. 2020, 6, 9–15. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Zhou, G.; Hao, T.; Xu, J.; Wang, J.; Qiu, C.; Prine, N.; Ali, J.; Feng, W.; et al. Efficient organic solar cell with 16.88% efficiency enabled by refined acceptor crystallization and morphology with improved charge transfer and transport properties. Adv. Energy Mater. 2020, 10, 1904234. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Hong, L.; Zhang, T.; Tang, Y.; Lin, B.; Xian, K.; Gao, B.; An, C.; Bi, P.; et al. Organic photovoltaic cell with 17% efficiency and superior processability. Natl. Sci. Rev. 2019, 7, 1239–1246. [Google Scholar] [CrossRef]
- Li, Z.; Yang, D.; Zhao, X.; Zhang, T.; Zhang, J.; Yang, X. Achieving an efficiency exceeding 10% for fullerene-based polymer solar cells employing a thick active layer via tuning molecular weight. Adv. Funct. Mater. 2017, 28, 1705257. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Vandewal, K.; Gadisa, A.; Oosterbaan, W.; Bertho, S.; Banishoeib, F.; Van Severen, I.; Lutsen, L.; Cleij, T.; Vanderzande, D.; Manca, J.V. The relation between open-circuit voltage and the onset of photocurrent generation by charge-transfer absorption in polymer: Fullerene bulk heterojunction solar cells. Adv. Funct. Mater. 2008, 18, 2064–2070. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Lin, Y.B.; Nugraha, M.I.; Firdaus, Y.; Scaccabarozzi, A.D.; Aniés, F.; Emwas, A.-H.; Yengel, E.; Zheng, X.; Liu, J.; Wahyudi, W.; et al. A simple n-dopant derived from diquat boosts the efficiency of organic solar cells to 18.3%. ACS Energy Lett. 2020, 5, 3663–3671. [Google Scholar] [CrossRef]
- Zhang, Q.; Bao, C.; Cui, S.; Zhong, P.; Zhang, K.; Zhu, W.; Liu, Y. Boosting the efficiency of PTB7-Th:PC71BM polymer solar cells via a low-cost halogen-free supramolecular solid additive. J. Mater. Chem. C 2020, 8, 16551–16560. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, Y.; Du, S.; Tong, J.; Shi, X.; Li, J.; Bao, X.; Xia, Y.; Liu, T.; et al. Significantly boosting efficiency of polymer solar cells by employing a nontoxic halogen-free additive. ACS Appl. Mater. Interfaces 2021, 13, 11117–11124. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Hwang, I.-W.; Lee, K. Top-down approach for nanophase reconstruction in bulk heterojunction solar cells. Adv. Mater. 2014, 26, 6275–6283. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, B.; Hu, B.; Xu, X.; Ma, W. Blade-cast nonfullerene organic solar cells in air with excellent morphology, efficiency, and stability. Adv. Mater. 2018, 30, 1800343. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Kim, J.K.; Kim, E.; Ahn, T.K.; Wang, D.H.; Park, J.H. Conflicted effects of a solvent additive on PTB7:PC71BM bulk heterojunction solar cells. J. Phys. Chem. C 2015, 119, 5954–5961. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.-T.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xu, T.; He, F.; Wang, W.; Wang, C.; Strzalka, J.; Liu, Y.; Wen, J.; Miller, D.J.; Chen, J.; et al. Hierarchical nano-morphologies promote exciton dissociation in polymer/fullerene bulk heterojunction solar cells. Nano Lett. 2011, 11, 3707–3713. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhao, W.; Tumbleston, J.R.; Wang, C.; Gu, Y.; Wang, D.; Briseno, A.L.; Ade, H.; Russell, T.P. Understanding the morphology of PTB7:PCBM Blends in organic photovoltaics. Adv. Energy Mater. 2013, 4, 1301377. [Google Scholar] [CrossRef]
- Hammond, M.R.; Kline, R.J.; Herzing, A.A.; Richter, L.J.; Germack, D.S.; Ro, H.-W.; Soles, C.L.; Fischer, D.A.; Xu, T.; Yu, L.; et al. Molecular order in high-efficiency polymer/fullerene bulk heterojunction solar cells. ACS Nano 2011, 5, 8248–8257. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yu, L. Understanding low bandgap polymer PTB7 and optimizing polymer solar cells based on it. Adv. Mater. 2014, 26, 4413–4430. [Google Scholar] [CrossRef]
- Supasai, T.; Amornkitbamrung, V.; Thanachayanont, C.; Tang, I.-M.; Sutthibutpong, T.; Rujisamphan, N. Visualizing nanoscale phase morphology for understanding photovoltaic performance of PTB7: PC71BM solar cell. Appl. Surf. Sci. 2017, 422, 509–517. [Google Scholar] [CrossRef]
- Foertig, A.; Kniepert, J.; Gluecker, M.; Brenner, T.; Dyakonov, V.; Neher, D.; Deibel, C. Nongeminate and geminate recombi-nation in PTB7:PC71BM solar cells. Adv. Funct. Mater. 2014, 24, 1306–1311. [Google Scholar] [CrossRef]
- Kniepert, J.; Lange, I.; Heidbrink, J.; Kurpiers, J.; Brenner, T.J.K.; Koster, L.J.A.; Neher, D. Effect of solvent additive on generation, recombination, and extraction in PTB7:PCBM solar cells: A conclusive experimental and numerical simulation study. J. Phys. Chem. C 2015, 119, 8310–8320. [Google Scholar] [CrossRef]
- Wen, G.; Hu, R.; Su, X.; Chen, Z.; Zhang, C.; Peng, J.; Zou, X.; He, X.; Dong, G.; Zhang, W. Excited-state properties of Y-series small molecule semiconductors. Dye. Pigm. 2021, 192, 109431. [Google Scholar] [CrossRef]
- Wen, G.; Zou, X.; Hu, R.; Peng, J.; Chen, Z.; He, X.; Dong, G.; Zhang, W. Ground- and excited-state characteristics in photo-voltaic polymer N2200. RSC Adv. 2021, 11, 20191–20199. [Google Scholar] [CrossRef]
- Su, X.; Zeng, X.; Nemec, H.; Zou, X.; Zhang, W.; Borgström, M.T.; Yartsev, A. Effect of hydrogen chloride etching on carrier recombination processes of indium phosphide nanowires. Nanoscale 2019, 11, 18550–18558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, X.; Li, C.; Su, X.; Liu, Y.; Finkelstein-Shapiro, D.; Zhang, W.; Yartsev, A. Carrier recombination processes in GaAs wafers passivated by wet nitridation. ACS Appl. Mater. Interfaces 2020, 12, 28360–28367. [Google Scholar] [CrossRef] [PubMed]
- Bencheikh, F.; Duché, D.; Ruiz, C.M.; Simon, J.-J.; Escoubas, L. Study of optical properties and molecular aggregation of con-jugated low band gap copolymers: PTB7 and PTB7-Th. J. Phys. Chem. C 2015, 119, 24643–24648. [Google Scholar] [CrossRef]
- Más-Montoya, M.; Janssen, R.A.J. The effect of H- and J-aggregation on the photophysical and photovoltaic properties of small thiophene-pyridine-DPP molecules for bulk-heterojunction solar cells. Adv. Funct. Mater. 2017, 27, 1605779. [Google Scholar] [CrossRef] [Green Version]
- Clarke, T.M.; Durrant, J.R. Charge photogeneration in organic solar cells. Chem. Rev. 2010, 110, 6736–6767. [Google Scholar] [CrossRef]
- Hedley, G.J.; Ruseckas, A.; Samuel, I.D.W. Light harvesting for organic photovoltaics. Chem. Rev. 2016, 117, 796–837. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Zhang, C.; Li, Q.; Zhang, Z.; Wang, X.; Xiao, M. Charge separation from an intra-moiety intermediate state in the high-performance PM6:Y6 organic photovoltaic blend. J. Am. Chem. Soc. 2020, 142, 12751–12759. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, H. Balancing charge generation and voltage loss toward efficient nonfullerene organic solar cells. Mater. Today Adv. 2020, 5, 100048. [Google Scholar] [CrossRef]
- Bhatta, R.S.; Tsige, M. Chain length and torsional dependence of exciton binding energies in P3HT and PTB7 conjugated polymers: A first-principles study. Polymer 2014, 55, 2667–2672. [Google Scholar] [CrossRef]
- Dkhissi, A. Excitons in organic semiconductors. Synth. Met. 2011, 161, 1441–1443. [Google Scholar] [CrossRef]
- Zhu, L.; Yi, Y.; Chen, L.; Shuai, Z. Exciton binding energy of electronic polymers: A first principles study. J. Theor. Comput. Chem. 2008, 7, 517–530. [Google Scholar] [CrossRef]
- Deibel, C.; Mack, D.; Gorenflot, J.; Schöll, A.; Krause, S.; Reinert, F.; Rauh, D.; Dyakonov, V. Energetics of excited states in the conjugated polymer poly(3-hexylthiophene). Phys. Rev. B 2010, 81, 085202. [Google Scholar] [CrossRef] [Green Version]
- Ma, M.; Ji, Q.; Yin, L.; Lin, K.; Wen, G.; Xu, Y.; Zhang, N.; Dong, G.; Yu, L.; Zhang, W.; et al. Core unit engineering of star-shaped acceptor polymers for all-polymer solar cells. Sol. Energy 2020, 207, 199–208. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, R.; Zeng, X.; Su, X.; Chen, Z.; Zou, X.; Peng, J.; Zhang, C.; Yartsev, A. Effect of post-thermal annealing on the performance and charge photogeneration dynamics of PffBT4T-2OD/PC71BM solar cells. Polymers 2019, 11, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolterfoht, M.; Philippa, B.; Armin, A.; Pandey, A.K.; White, R.D.; Burn, P.L.; Meredith, P.; Pivrikas, A. Advantage of sup-pressed non-langevin recombination in low mobility organic solar cells. Appl. Phys. Lett. 2014, 105, 013302. [Google Scholar] [CrossRef] [Green Version]
- Hughes, M.P.; Rosenthal, K.D.; Ran, N.A.; Seifrid, M.; Bazan, G.C.; Nguyen, T.-Q. Determining the dielectric constants of organic photovoltaic materials using impedance spectroscopy. Adv. Funct. Mater. 2018, 28, 1801542. [Google Scholar] [CrossRef]







| Active Layers | Jsc (mA/cm2) | Voc (V) | FF | PCE (%) | PCEmax (%) |
|---|---|---|---|---|---|
| PTB7 | 0.32 ± 0.06 | 0.68 ± 0.02 | 0.49 ± 0.02 | 0.11 ± 0.02 | 0.13 |
| PTB7:PC71BM | 11.26 ± 0.29 | 0.70 ± 0.01 | 0.46 ± 0.01 | 3.63 ± 0.10 | 3.73 |
| DIO-treated PTB7:PC71BM | 17.01 ± 0.25 | 0.71 ± 0.04 | 0.68 ± 0.02 | 8.21 ± 0.15 | 8.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Hu, R.; Wen, G.; Zou, X.; Qing, M.; Peng, J.; He, X.; Zhang, W. Understanding of Photophysical Processes in DIO Additive-Treated PTB7:PC71BM Solar Cells. Crystals 2021, 11, 1139. https://doi.org/10.3390/cryst11091139
Su X, Hu R, Wen G, Zou X, Qing M, Peng J, He X, Zhang W. Understanding of Photophysical Processes in DIO Additive-Treated PTB7:PC71BM Solar Cells. Crystals. 2021; 11(9):1139. https://doi.org/10.3390/cryst11091139
Chicago/Turabian StyleSu, Xiaojun, Rong Hu, Guanzhao Wen, Xianshao Zou, Mengyao Qing, Jun Peng, Xiaochuan He, and Wei Zhang. 2021. "Understanding of Photophysical Processes in DIO Additive-Treated PTB7:PC71BM Solar Cells" Crystals 11, no. 9: 1139. https://doi.org/10.3390/cryst11091139
APA StyleSu, X., Hu, R., Wen, G., Zou, X., Qing, M., Peng, J., He, X., & Zhang, W. (2021). Understanding of Photophysical Processes in DIO Additive-Treated PTB7:PC71BM Solar Cells. Crystals, 11(9), 1139. https://doi.org/10.3390/cryst11091139