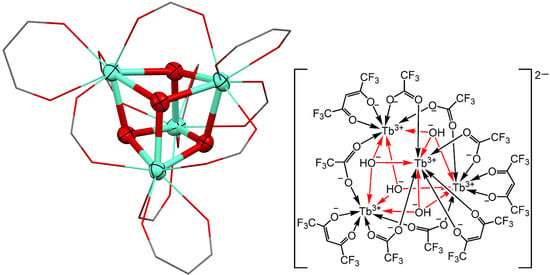
[Tb4(OH)4]-Cuboid Complex Dianion Stabilized with Six Carboxylate Bridges and Four Diketonate Caps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation
2.2. Structural Analysis
2.3. Magnetic Measurements
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord. Chem. Rev. 2015, 289, 74–122. [Google Scholar] [CrossRef]
- Demir, S.; Jeon, I.R.; Long, J.R.; Harris, T.D. Radical ligandcontaining single-molecule magnets. Coord. Chem. Rev. 2015, 289, 149–176. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Chen, Y.C.; Tong, M.L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Kahn, O. Molecular Magnetism; VCH: New York, NY, USA, 1993. [Google Scholar]
- Caneschi, A.; Gatteschi, D.; Sessoli, R.; Rey, P. Toward molecular magnets: The metal-radical approach. Acc. Chem. Res. 1989, 22, 392–398. [Google Scholar] [CrossRef]
- Ishida, T.; Ito, S.; Homma, Y.; Kyoden, Y. Molecular S = 2 High-Spin, S = 0 Low-Spin and S = 0 ⇄ 2 Spin-Transition/-Crossover Nickel(II)-Bis(nitroxide) Coordination Compounds. Inorganics 2021, 9, 10. [Google Scholar] [CrossRef]
- Peng, Y.; Powell, A.K. What do 3d-4f butterflies tell us? Coord. Chem. Rev. 2021, 426, 213490. [Google Scholar] [CrossRef]
- Watanabe, R.; Fujiwara, K.; Okazawa, A.; Tanaka, G.; Yoshii, S.; Nojiri, H.; Ishida, T. Chemical trend of Ln−M exchange couplings in heterometallic complexes with Ln = Gd, Tb, Dy, Ho, Er and M = Cu, V. Chem. Commun. 2011, 47, 2110–2112. [Google Scholar] [CrossRef]
- Ikegaya, N.; Kanetomo, T.; Murakami, R.; Ishida, T. Triply Radical-coordinated Gadolinium(III) Complex as a High-spin S = 5 Assembly. Chem. Lett. 2012, 41, 82–83. [Google Scholar] [CrossRef]
- Kanetomo, T.; Naoi, Y.; Enomoto, M. Gadolinium-Triradical Complex with Ground S = 10 State: Synthesis, Structural Characterization and Magnetic Studies. Eur. J. Inorg. Chem. 2021, 2021, 1130–1136. [Google Scholar] [CrossRef]
- Nakamura, T.; Kanetomo, T.; Ishida, T. Strong Antiferromagnetic Interaction in a Gadolinium(III) Complex with Methoxy-TEMPO Radical: A Relation between the Coupling and the Gd–O–N Angle. Inorg. Chem. 2020, 60, 535–539. [Google Scholar] [CrossRef]
- Escobar, L.B.L.; Guedes, G.P.; Soriano, S.; Speziali, N.L.; Jordão, A.K.; Cunha, A.C.; Ferreira, V.F.; Maxim, C.; Novak, M.A.; Andruh, M.; et al. New Families of Hetero-tri-spin 2p–3d–4f Complexes: Synthesis, Crystal Structures, and Magnetic Properties. Inorg. Chem. 2014, 53, 7508–7517. [Google Scholar] [CrossRef]
- Novitchi, G.; Shova, S.; Lan, Y.; Wernsdorfer, W.; Train, C. Verdazyl Radical, a Building Block for a Six-Spin-Center 2p–3d–4f Single-Molecule Magnet. Inorg. Chem. 2016, 55, 12122–12125. [Google Scholar] [CrossRef]
- Li, H.; Sun, J.; Yang, M.; Sun, Z.; Tang, J.; Ma, Y.; Li, L. Functionalized nitronyl nitroxide biradicals for the construction of 3d–4f heterometallic compounds. Inorg. Chem. 2018, 57, 9757–9765. [Google Scholar] [CrossRef]
- Vaz, M.G.F.; Andruh, M. Molecule-based magnetic materials constructed from paramagnetic organic ligands and two different metal ions. Coord. Chem. Rev. 2021, 427, 213611. [Google Scholar] [CrossRef]
- Patrascu, A.A.; Calancea, S.; Briganti, M.; Soriano, S.; Madalan, A.M.; Cassaro, R.A.A.; Caneschi, A.; Totti, F.; Vaz, M.G.F.; Andruh, M. A chimeric design of heterospin 2p–3d, 2p–4f, and 2p–3d–4f complexes using a novel family of paramagnetic dissymmetric compartmental ligands. Chem. Commun. 2017, 53, 6504–6507. [Google Scholar] [CrossRef]
- Parr, R.G.; Pearson, R.G. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Kudyakova, Y.S.; Slepukhin, P.A.; Valova, M.S.; Burgart, Y.V.; Saloutin, V.I.; Bazhin, D.N. Role of alkyl substituents in the structure and luminescence properties of discrete terbium(III)-lithium(I) Β-Diketonates. J. Mol. Struct. 2021, 1226, 129331. [Google Scholar] [CrossRef]
- Takano, R.; Ishida, T. Polymeric Terbium(III) Squarate Hydrate as a Luminescent Magnet. Crystals 2021, 11, 1221. [Google Scholar] [CrossRef]
- Helbert, J.N.; Kopf, P.W.; Poindexter, E.H.; Wagner, B.E. Complexing and protonation of free-radical imidazolin-1-oxyl and imidazolin-1-oxyl 3-oxide ligands: A magnetic-resonance investigation. J. Chem. Soc. Dalton Trans. 1975, 1975, 998–1006. [Google Scholar] [CrossRef]
- Kanetomo, T.; Yoshitake, T.; Ishida, T. Strongest Ferromagnetic Coupling in Designed Gadolinium(III)–Nitroxide Coordination Compounds. Inorg. Chem. 2016, 55, 8140–8146. [Google Scholar] [CrossRef]
- Kanetomo, T.; Ishida, T. Preparation and characterization of [Gd(hfac)3(DTBN)(H2O)] (DTBN = di-t-butyl nitroxide). Ferromagnetic Gd3+–Gd3+ super–superexchange. Chem. Commun. 2014, 50, 2529–2531. [Google Scholar] [CrossRef]
- Richardson, M.F.; Wagner, W.F.; Sands, D.E. Rare-earth trishexafluoroacetylacetonates and related compounds. J. Inorg. Nucl. Chem. 1968, 30, 1275–1289. [Google Scholar] [CrossRef]
- Bagryanskaya, I.Y.; Politanskaya, L.V.; Tretyakov, E.V. Frequently used, but still unknown: Terbium(III) tris-hexafluoroacetylacetonate dihydrate. Inorg. Chem. Commun. 2016, 66, 47–50. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar]
- Eliseeva, S.V.; Pleshkov, D.N.; Lyssenko, K.A.; Lepnev, L.S.; Bünzli, J.C.G.; Kuzmina, N.P. Highly luminescent and triboluminescent coordination polymers assembled from lanthanide β-diketonates and aromatic bidentate O-donor ligands. Inorg. Chem. 2010, 49, 9300–9311. [Google Scholar] [PubMed]
- Lluncll, M.; Casanova, D.; Circra, J.; Bofill, J.M.; Alcmany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE, Version 2.1; University of Barcelona: Barcelona, Spain; Hebrew University of Jerusalem: Jerusalem, Israel, 2005. [Google Scholar]
- Li, X.; Sun, H.L.; Wu, X.S.; Qiu, X.; Du, M. Unique (3,12)-connected porous lanthanide–organic frameworks based on Ln4O4 clusters: Synthesis, crystal structures, luminescence, and magnetism. Inorg. Chem. 2010, 49, 1865–1871. [Google Scholar] [CrossRef]
- Wong, H.Y.; Chan, W.T.K.; Law, G.L. Assembly of lanthanide(III) cubanes and dimers with single-molecule magnetism and photoluminescence. Inorg. Chem. 2018, 57, 6893–6902. [Google Scholar] [PubMed]
- Andrews, P.C.; Gee, W.J.; Junk, P.C.; MacLellan, J.G. Systematic study of the formation of the lanthanoid cubane cluster motif mediated by steric modification of diketonate ligands. Dalton Trans. 2011, 40, 12169–12179. [Google Scholar] [CrossRef]
- Wu, Y.; Morton, S.; Kong, X.; Nichol, G.S.; Zheng, Z. Hydrolytic synthesis and structural characterization of lanthanide-acetylacetonato/hydroxo cluster complexes—A systematic study. Dalton Trans. 2011, 40, 1041–1046. [Google Scholar] [CrossRef] [Green Version]
- Luneau, D.; Rey, P.; Laugier, J.; Belorizky, E.; Cogne, A. Ferromagnetic behavior of nickel(II)–imino nitroxide derivatives. Inorg. Chem. 1992, 31, 3578–3584. [Google Scholar] [CrossRef]
- Okazawa, A. Magneto-Structural Relationship on Strong Exchange Interactions between Chelating Nitroxide Radical and Transition-Metal Spins. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012002. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Ishida, T. Spin-Parity Behavior in the Exchange-Coupled Lanthanoid-Nitroxide Molecular Magnets. IOP Conf. Ser. Mater. Sci. Eng. 2017, 202, 012001. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamaguchi, Y.; Ishida, T. [Tb4(OH)4]-Cuboid Complex Dianion Stabilized with Six Carboxylate Bridges and Four Diketonate Caps. Crystals 2022, 12, 402. https://doi.org/10.3390/cryst12030402
Yamaguchi Y, Ishida T. [Tb4(OH)4]-Cuboid Complex Dianion Stabilized with Six Carboxylate Bridges and Four Diketonate Caps. Crystals. 2022; 12(3):402. https://doi.org/10.3390/cryst12030402
Chicago/Turabian StyleYamaguchi, Yoshiki, and Takayuki Ishida. 2022. "[Tb4(OH)4]-Cuboid Complex Dianion Stabilized with Six Carboxylate Bridges and Four Diketonate Caps" Crystals 12, no. 3: 402. https://doi.org/10.3390/cryst12030402
APA StyleYamaguchi, Y., & Ishida, T. (2022). [Tb4(OH)4]-Cuboid Complex Dianion Stabilized with Six Carboxylate Bridges and Four Diketonate Caps. Crystals, 12(3), 402. https://doi.org/10.3390/cryst12030402