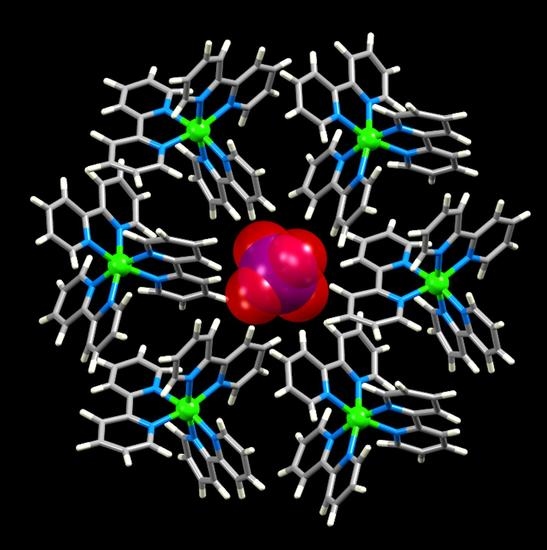
Embracing [XY3]m– and [XY4]m– Anions in Salts of [M(bpy)3]q+
Abstract
:1. Introduction
2. Methods
3. Defining the Sets of Structures for Analysis
4. An Aside on Chirality
5. [M(bpy)3]q+ Combined with [XY3]m–
6. [M(bpy)3]q+ Combined with [XY4]m–
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blau, F. Die Destillation pyridinmonocarbonsaurer Salze. Ber. Dtsch. Chem. Ges. 1888, 21, 1077–1078. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. The Early Years of 2,2′-Bipyridine—A Ligand in Its Own Lifetime. Molecules 2019, 24, 3951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaes, C.; Katz, A.; Hosseini, M.W. Bipyridine: The Most Widely Used Ligand. A Review of Molecules Comprising at Least Two 2,2′-Bipyridine Units. Chem. Rev. 2000, 100, 3553–3590. [Google Scholar] [CrossRef] [PubMed]
- Summers, L.A. The Bipyridines. Adv. Heterocycl. Chem. 1984, 35, 281–374. [Google Scholar] [CrossRef]
- Constable, E.C. Homoleptic Complexes of 2,2′-Bipyridine. Adv. Inorg. Chem. 1989, 34, 1–63. [Google Scholar] [CrossRef]
- Lindoy, L.F.; Livingstone, S.E. Complexes of iron(II), cobalt(II) and nickel(II) with α-diimines and related bidentate ligands. Coord. Chem. Rev. 1967, 2, 173–193. [Google Scholar] [CrossRef]
- McWhinnie, W.R.; Miller, J.D. The Chemistry of Complexes Containing 2,2′-Bipyridyl, 1,10-Phenanthroline, or 2,2′,6′,2′′-Terpyridyl as Ligands. Adv. Inorg. Chem. Radiochem. 1970, 12, 135–215. [Google Scholar] [CrossRef]
- Brandt, W.W.; Dwyer, F.P.; Gyarfas, E.D. Chelate Complexes of 1,10-Phenanthroline and Related Compounds. Chem. Rev. 1954, 54, 959–1017. [Google Scholar] [CrossRef]
- Smith, A.P.; Fraser, C.L. Bipyridine Ligands. In Comprehensive Coordination Chemistry, 2nd ed.; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Oxford, UK, 2003; pp. 1–23. [Google Scholar] [CrossRef]
- Grice, K.A.; Nganga, J.K.; Naing, M.D.; Angeles-Boza, A.M. Bipyridine Ligands. In Comprehensive Coordination Chemistry, 3rd ed.; Constable, E.C., Parkin, G., Que, L., Eds.; Elsevier: Oxford, UK, 2021; pp. 60–77. [Google Scholar] [CrossRef]
- Schilt, A.A. Analytical Applications of 1,10-Phenanthroline and Related Compounds. In International Series of Monographs on Analytical Chemistry; Pergamon: Oxford, UK, 1969; Volume 32, pp. 10–53. [Google Scholar] [CrossRef]
- McKenzie, E.D. The steric effect in bis(2,2′-bipyridyl) and bis(1,10-phenanthroline) metal compounds. Coord. Chem. Rev. 1971, 6, 187–216. [Google Scholar] [CrossRef]
- Constable, E.C. Forty years on—Covalent hydration of transition metal complexes revisited. Polyhedron 2016, 103B, 295–306. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. More hydra than Janus—Non-classical coordination modes in complexes of oligopyridine ligands. Coord. Chem. Rev. 2017, 350, 84–104. [Google Scholar] [CrossRef] [Green Version]
- Constable, E.C.; Housecroft, C.E. ‘Simple’ Oligopyridine Complexes—Sources of Unexpected Structural Diversity. Aust. J. Chem. 2020, 73, 390–398. [Google Scholar] [CrossRef]
- Dance, I.; Scudder, M. Supramolecular motifs: Sextuple aryl embraces in crystalline [M(2,2′-bipy)3] and related complexes. J. Chem. Soc. Dalton Trans. 1998, 1341–1350. [Google Scholar] [CrossRef]
- Dance, I. Intermolecular Embraces and Intermolecular Energies. Mol. Cryst. Liq. Cryst. 2006, 440, 265–293. [Google Scholar] [CrossRef]
- Dance, I. Inorganic intermolecular motifs, and their energies. CrystEngComm 2003, 5, 208–221. [Google Scholar] [CrossRef]
- Dance, I. Distance criteria for crystal packing analysis of supramolecular motifs. New J. Chem. 2003, 27, 22–27. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. Halide Ion Embraces in Tris(2,2′-bipyridine)metal Complexes. Crystals 2020, 10, 671. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Ruiz-Pérez, C.; Lorenzo Luis, P.A.; Lloret, F.; Julve, M. Dimensionally controlled hydrogen-bonded nanostructures: Synthesis, structure, thermal and magnetic behaviour of the tris-(chelated)nickel(II) complex [Ni(bipy)3Cl2].5.5H2O (bipy = 2,2′-bipyridyl). Inorg. Chim. Acta 2002, 336, 131–136. [Google Scholar] [CrossRef]
- Bruno, I.J.; Cole, J.C.; Edgington, P.R.; Kessler, M.; Macrae, C.F.; McCabe, P.; Pearson, J.; Taylor, R. New software for searching the Cambridge Structural Database and visualising crystal structures. Acta Cryst. 2002, B58, 389–397. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- Thuéry, P.; Harrowfield, J. Uranyl Ion Complexes with 1,1′-Biphenyl-2,2′,6,6′-tetracarboxylic Acid: Structural and Spectroscopic Studies of One- to Three-Dimensional Assemblies. Inorg. Chem. 2015, 54, 6296–6305. [Google Scholar] [CrossRef]
- Handke, M.; Wu, Y.; Li, Y.; Hu, C.T.; Ward, M.D. Encapsulation of the [Ru(bpy)3]2+ luminophore in a unique hydrogen-bonded host framework. CrystEngComm 2020, 22, 3749–3752. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Topchiyan, P.; Baidina, I.; Korolkov, I.; Filatov, E.; Zvereva, V.; Plyusnin, P.; Slavinskaya, E.; Gerasimov, E. Double complex salts containing [Pt(NO3)6]2− anion and Rh(III) complex cations: Synthesis, structure and utilisation for preparing (Rh–Pt)/CeO2 catalysts. J. Mol. Struct. 2020, 1211, 128108. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Staszak, Z.; Bronowska, W.; Pietraszko, A.; Cieślak-Golonka, M. Crystal structure and 4.2 K electronic spectrum of [Co(bpy)3](CrO4)0.5NO3·7H2O, a double salt containing uncoordinated chromate ion. J. Mol. Struct. 2003, 654, 197–204. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Bronowska, W.; Pietraszko, A.; Staszak, Z.; Cieślak-Golonka, M. Synthesis, crystal structure and spectroscopic characterisation of double salt [Zn(bpy)3](CrO4)0.5NO36.5H2O. J. Mol. Struct. 2002, 608, 151–160. [Google Scholar] [CrossRef]
- Jaeger, F.M.; van Dijk, J.A. On some complex dipyridyl-salts of nickel and copper. J. Proc. K. Ned. Akad. Wet. B 1935, 38, 972–977. [Google Scholar]
- Henling, L.M.; Marsh, R.E. Some more space-group corrections. Acta Crystallogr. Sect. C 2014, 70, 834–836. [Google Scholar] [CrossRef]
- Zhuge, F.; Wu, B.; Liang, J.; Yang, J.; Liu, Y.; Jia, C.; Janiak, C.; Tang, N.; Yang, X.-J. Full- or Half-Encapsulation of Sulfate Anion by a Tris(3-pyridylurea) Receptor: Effect of the Secondary Coordination Sphere. Inorg. Chem. 2009, 48, 10249–10256. [Google Scholar] [CrossRef]
- Mustafina, A.R.; Skripacheva, V.V.; Gubaidullin, A.T.; Latipov, S.K.; Toropchina, A.V.; Yanilkin, V.V.; Solovieva, S.E.; Antipin, I.S.; Konovalov, A.I. Outer-Sphere Association of p-Sulfonatothiacalix[4]arene and Tetrasulfonatomethylated Calix[4]resorcinarene with Cobalt(III) Tris(dipyridyl): The Effect on the Spectral and Electrochemical Properties of the Latter. Inorg. Chem. 2005, 44, 4017–4023. [Google Scholar] [CrossRef]
- Yu, K.; Chen, W.-L.; Zhou, B.-B.; Li, Y.-G.; Yu, Y.; Su, Z.-H.; Gao, S.; Chen, Y. New extended poly(oxomolybdophosphates) based on strontium(II) linkers. CrystEngComm 2011, 13, 3417–3424. [Google Scholar] [CrossRef]
- Scott, R.T.W.; Parsons, S.; Murugesu, M.; Wernsdorfer, W.; Christou, G.; Brechin, E.K. Linking Centered Manganese Triangles into Larger Clusters: A {Mn32} Truncated Cube. Angew. Chem. Int. Ed. 2005, 44, 6540–6543. [Google Scholar] [CrossRef]
- Andres, R.; Brissard, M.; Gruselle, M.; Train, C.; Vaissermann, J.; Malezieux, B.; Jamet, J.P.; Verdaguer, M. Rational Design of Three-Dimensional (3D) Optically Active Molecule-Based Magnets: Synthesis, Structure, Optical and Magnetic Properties of {[Ru(bpy)3]2+, ClO4−, [MnIICrIII(ox)3]−}n and {[Ru(bpy)2ppy]+, [MIICrIII(ox)3]−}n, with MII = MnII, NiII. X-ray Structure of {[ΔRu(bpy)3]2+, ClO4−, [ΔMnIIΔCrIII(ox)3]−}n and {[ΛRu(bpy)2ppy]+, [ΛMnIIΛCrIII(ox)3]−}n. Inorg. Chem. 2001, 40, 4633–4640. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Agudo, J.M. Molecule-Based Magnets Formed by Bimetallic Three-Dimensional Oxalate Networks and Chiral Tris(bipyridyl) Complex Cations. The Series [ZII(bpy)3][ClO4][MIICrIII(ox)3] (ZII = Ru, Fe, Co, and Ni; MII = Mn, Fe, Co, Ni, Cu, and Zn; ox = Oxalate Dianion). Inorg. Chem. 2001, 40, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Molina, M.; Lloret, F.; Ruiz-Pérez, C.; Julve, M. Weak Ferromagnetism in Chiral 3-Dimensional Oxalato-Bridged Cobalt(II) Compounds. Crystal Structure of [Co(bpy)3][Co2(ox)3]ClO4. Inorg. Chem. 1998, 37, 4131–4135. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Pellaux, R.; Schneuwly, P.; Hauser, A. Chiral, Three-Dimensional Supramolecular Compounds: Homo- and Bimetallic Oxalate- and 1,2-Dithiooxalate-Bridged Networks. A Structural and Photophysical Study. Inorg. Chem. 1996, 35, 1451–1460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stebler, M.; Gutierrez, A.; Ludi, A.; Burgi, H.-B. Synthesis and crystal structure of tris(2,2′-bipyridine)rhenium(2+) perrhenate. Inorg. Chem. 1987, 26, 1449–1451. [Google Scholar] [CrossRef]
- Fang, Y.; Jie, Q.; Huang, C. Synthesis and Crystal Structure of a Keggin-Polymolybdate Complex {PMoVI7MoV4VVO40(VIVO)2[Co(bipy)2(H2O)]2}[Co(bipy)3]2{PMoVI7MoV4VVO40(VIVO)2}.6H2O. Beihua Daxue Xuebao Ziran Kexueban 2010, 11, 213–217. [Google Scholar]
- Jacobs, G.; Speeke, F. The unit cell and space group of the complex tridipyridyl-nickel sulfate. Acta Cryst. 1955, 8, 67–68. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E. When Stereochemistry Raised Its Ugly Head in Coordination Chemistry—An Appreciation of Howard Flack. Chemistry 2020, 2, 759–776. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology, 2nd ed.; IUPAC Gold Book; Online Version (2019-) created by Chalk, S.J.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]
- Connelly, N.G.; Damhus, T.; Hartshorn, R.M.; Hutton, A.T. (Eds.) Nomenclature of Inorganic Chemistry, IUPAC Recommendations 2005; IUPAC Red Book; RSC Publishing: Cambridge, UK, 2005. [Google Scholar]
- Luo, Q.; Chen, Y.-Q.; Peng, H.-Q.; Ji, Q.; Wang, X.-X.; Wei, L.; Zhong, Q.-Y.; Chen, W.-T. Preparation, characterization, and photoluminescent and semiconductive properties of an iron compound. Inorg. Nano-Metal Chem. 2022. [Google Scholar] [CrossRef]
- Lin, J.-M.; Chen, W.-B.; Lin, X.-M.; Lin, A.-H.; Ma, C.-Y.; Dong, W.; Tian, C.-E. Syntheses, structures and multi-photoluminescence images with confocal microscopy for three 5,5′-azotetrazolate (AZT) based Zn(II) and Ni(II) complexes. Chem. Commun. 2011, 47, 2402–2404. [Google Scholar] [CrossRef] [PubMed]
- Evstifeev, I.; Kiskin, M.; Eremenko, I. Tris(2,2′-bipyridine)-cobalt(ii) dinitrate acetonitrile solvate monohydrate. CSD Commun. 2012. [Google Scholar] [CrossRef]
- Shen, L.; Han, Z.; Brennessel, W.W.; Holland, P.L.; Eisenberg, R. Tris(2,2′-bipyridine)-nickel(ii) dinitrate acetonitrile solvate monohydrate. CSD Commun. 2012. [Google Scholar] [CrossRef]
- Kumar, D.; Kapoor, I.P.S.; Singh, G.; Goel, N.; Singh, U.P. Preparation, X-ray crystallography and thermolysis of transition metal nitrates of 2,2′-bipyridine (Part 63). J. Therm. Anal. Calorim. 2012, 107, 325–334. [Google Scholar] [CrossRef]
- Tomić, Z.; Prelesnik, B.; Karanović, L.; Poleti, D. Crystal structure of di-[tris(2,2′-bipyridine)nickel(II)] hydrogen phthalate trinitrate tetrahydrate. J. Serb. Chem. Soc. 1996, 61, 97–106. [Google Scholar]
- Miao, X.-H.; Zhu, L.-G. Synthesis, supramolecular structures and catalytic properties of nickel(II) 3-sulfobenzoate complexes with chelating amine ligands. CrystEngComm 2009, 11, 2500–2509. [Google Scholar] [CrossRef]
- Jurić, M.; Planinić, P.; Brničević, N.; Milić, D.; Matković-Čalogović, D.; Pajić, D.; Zadro, K. New Heterometallic (CuII and CrIII) Complexes—First Crystal Structure of an Oxalate-Bridged Ferromagnetically Coupled [CuIICrIIICuII] System. Eur. J. Inorg. Chem. 2006, 2006, 2701–2710. [Google Scholar] [CrossRef]
- Jurić, M.; Planinić, P.; Žilić, D.; Rakvin, B.; Prugovečki, B.; Matković-Čalogović, D. A new heterometallic (Ni2+ and Cr3+) complex—Crystal structure and spectroscopic characterization. J. Mol. Struct. 2009, 924, 73–80. [Google Scholar] [CrossRef]
- Muzioł, T.M.; Tereba, N.; Podgajny, R.; Kędziera, D.; Wrzeszcz, G. Solvent-assisted structural conversion involving bimetallic complexes based on the tris(oxalato) ferrate(III) unit with the green → blue → red crystal color sequence. Dalton Trans. 2019, 48, 11536–11546. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; You, W.; Huang, W. Tris(2,2′-bipyridyl-κ2N,N′)copper(II) sulfate 7.5-hydrate. Acta Crystallogr. Sect. E 2009, 65, m129–m130. [Google Scholar] [CrossRef] [PubMed]
- Avdeeva, V.V.; Vologzhanina, A.V.; Goeva, L.V.; Malinina, E.A.; Kuznetsov, N.T. Boron Cluster Anions [BnHn]2– (n = 10, 12) in Reactions of Iron(II) and Iron(III) Complexation with 2,2′-Bipyridyl and 1, 10-Phenanthroline. Z. Anorg. Allg. Chem. 2014, 640, 2149–2160. [Google Scholar] [CrossRef]
- Li, Y.-L.; Yang, Y.-Q.; Mao, F.-F.; Chen, Z.-M. Synthesis, Crystal Structure, and Fluorescent and Thermal Stability Properties of Two New Cobalt(II) Complexes with 2,2′-Bipy as Ligand. Chin. J. Struct. Chem. 2019, 38, 769–776. [Google Scholar] [CrossRef]
- Wada, A.; Sakabe, N.; Tanaka, J. The crystal structure of tris(2,2′-bipyridyl)nickel(II) sulphate hydrate, [Ni(C10H8N2)3]SO4.7.5H2O. Acta Crystallogr. Sect. B 1976, 32, 1121–1127. [Google Scholar] [CrossRef]
- Eom, G.H.; Park, H.M.; Hyun, M.Y.; Jang, S.P.; Kim, C.; Lee, J.H.; Lee, S.J.; Kim, S.-J.; Kim, Y. Anion effects on the crystal structures of ZnII complexes containing 2,2′-bipyridine: Their photoluminescence and catalytic activities. Polyhedron 2011, 30, 1555–1564. [Google Scholar] [CrossRef]
- Huang, Q.; Diao, L.; Zhang, C.; Lei, F. Cyclic Water Clusters in Tape-Like and Cage-Like Structures. Molecules 2011, 16, 2871–2883. [Google Scholar] [CrossRef] [Green Version]
- Freire, E.; Baggio, S.; Mombru, A.; Baggio, R. Tris(2,2′-bipyridyl-N,N′)nickel(II) thiosulfate heptahydrate. Acta Crystallogr. Sect. C 2000, 56, 541–543. [Google Scholar] [CrossRef]
- Baggio, S.; Pardo, M.I.; Baggio, R.; Garland, M.T. Tris(2,2′-bipyridyl-N,N′)zinc(II) Thiosulfate Heptahydrate. Acta Crystallogr. Sect. C 1997, 53, 1570–1572. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Pietraszko, A.; Bronowska, W.; Staszak, Z.; Jezierska, J.; Cieślak-Golonka, M. Geometric distortions of octahedral cations and tetrahedral anions in disordered [Cu(bpy)3]CrO4.7.5H2O crystal—A comparative study. Polyhedron 2010, 29, 2574–2581. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Staszak, Z.; Bronowska, W.; Pietraszko, A.; Cieślak-Golonka, M. Spectroscopic and structural studies of chromate ions in zinc complexes with 2,2′-bipyridine. Analysis of the lowest triplet states in the CrO42− entity. Polyhedron 2001, 20, 2063–2072. [Google Scholar] [CrossRef]
- Pietraszko, A.; Bronowska, W.; Wojciechowska, A.; Staszak, Z.; Cieślak-Golonka, M. One-, Two- and Non-Coordinated CrO42– Entity in the Nickel(II) Complexes. Structural and Spectroscopic Investigation. Pol. J. Chem. 2002, 76, 309–324. [Google Scholar]
- Zeng, Q.-X.; Yang, G.-Y.; Xu, J.-Q. Hydrothermal synthesis and crystal structure of [Ni(2,2’-bpy)3]MoO4.7.5H2O. Jiegou Huaxue 2003, 22, 336–340. [Google Scholar]
- Cheung, E.Y.; Fujii, K.; Guo, F.; Harris, K.D.M.; Hasebe, S.; Kuroda, R. Structural Chemistry of a New Chiral Anhydrous Phase of Ru(bipy)3(ClO4)2 Established from Powder X-ray Diffraction Analysis. Cryst. Growth Des. 2011, 11, 3313–3317. [Google Scholar] [CrossRef]
- Fernández, G.; Corbella, M.; Alfonso, M.; Stoeckli-Evans, H.; Castro, I. A Comparative XAS and X-ray Diffraction Study of New Binuclear Mn(III) Complexes with Catalase Activity. Indirect Effect of the Counteranion on Magnetic Properties. Inorg. Chem. 2004, 43, 6684–6698. [Google Scholar] [CrossRef] [PubMed]
- Sohncke, L. Entwickelung Einer Theorie der Krystallstruktur; B.G. Teubner: Leipzig, Germany, 1879. [Google Scholar]
- Krausz, E.; Riesen, H.; Rae, A.D. Crystal Structures of and Polarized Absorption-Spectroscopy in Racemic and Resolved [Ru(bpy)3](ClO4)2 and [Zn(bpy)3](ClO4)2 Bpy = 2,2′-Bipyridine. Aust. J. Chem. 1995, 48, 929–954. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Sobolev, A.N. The Crystal Structure of Tris(2,2′-bipyridine)ruthenium(II) Perchlorate. Aust. J. Chem. 1994, 47, 763–767. [Google Scholar] [CrossRef]
- Moore, C.; Grotjahn, D.; Rheingold, A. Tris(2,2′-Bipyridine)-ruthenium(ii) diperchlorate. CSD Commun. 2010. [Google Scholar] [CrossRef]
- Klement, U.; Trumbach, D.; Yersin, H. Crystal structure of tris(2,2′-bipyridyl)-zinc(II) Perchlorate, (Zn(C10H8N2)3)(ClO4)2. Z. Kristallogr.—Cryst. Mater. 1995, 210, 228. [Google Scholar] [CrossRef]
- Chen, X.-M.; Wang, R.-Q.; Yu, X.-L. Tris(2,2′-bipyridine)zinc(II) Perchlorate. Acta Crystallogr. Sect. C 1995, 51, 1545–1547. [Google Scholar] [CrossRef]
- Yao, J.-C.; Ma, L.-F.; Yao, F.-J. Crystal structure of tris(2,2′-bipyridine)cobalt(II) diperchlorate, [Co(C10H8N2)3][ClO4]2. Z. Kristallogr.-N. Cryst. Struct. 2005, 220, 483–484. [Google Scholar] [CrossRef]
- Batten, S.R.; Murray, K.S.; Sinclair, N.J. Tris(2,2′-bipyridyl-N,N′)iron(II) diperchlorate. Acta Cryst. 2000, C56, e320. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, X.; Xu, Y.; Cao, R.; Hong, M. Tris(2,2′-bipyridine)nickel(II) diperchlorate. Acta Cryst. 2003, E59, m300–m302. [Google Scholar] [CrossRef]
- Sangeeta, S. Tris(2,2′-bipyridine)nickel(ii) diperchlorate. CSD Commun. 2017. [Google Scholar] [CrossRef]
- Kawamata, Y.; Vantourout, J.C.; Hickey, D.P.; Bai, P.; Chen, L.; Hou, Q.; Qiao, W.; Barman, K.; Edwards, M.A.; Garrido-Castro, A.F.; et al. Electrochemically Driven, Ni-Catalyzed Aryl Amination: Scope, Mechanism, and Applications. J. Am. Chem. Soc. 2019, 141, 6392–6402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pointillart, F.; Train, C.; Villain, F.; dit Moulin, C.C.; Gredin, P.; Chamoreau, L.-M.; Gruselle, M.; Aullon, G.; Alvarez, S.; Verdaguer, M. Six-fold Oxygen-Coordinated Triplet (S = 1) Palladium(II) Moieties Templated by Tris(bipyridine)ruthenium(II) Ions. J. Am. Chem. Soc. 2007, 129, 1327–1334. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, D.J.; Haushalter, R.C.; Zubieta, J. The hydrothermal synthesis and structural characterization of a new class of compounds: Nickel organoamine-halocadmates. Inorg. Chim. Acta 1999, 292, 41–51. [Google Scholar] [CrossRef]
- Lin, S.-H.; Wang, Z.-K.; Zhang, B.-H.; Hu, H.-M.; Huang, J.-S. Crystal structure of tris(2,2’-bipyridine)nickel(II) tetrachlorozincate. Jiegou Huaxue 2000, 19, 95–98. [Google Scholar]
- Eckenhoff, W.T.; Biernesser, A.B.; Pintauer, T. Structural characterization and investigation of iron(III) complexes with nitrogen and phosphorus based ligands in atom transfer radical addition (ATRA). Inorg. Chim. Acta 2012, 382, 84–95. [Google Scholar] [CrossRef]
- Dorval, C.; Tricoire, M.; Begouin, J.-M.; Gandon, V.; Gosmini, C. Cobalt-Catalyzed C(sp2)–CN Bond Activation: Cross-Electrophile Coupling for Biaryl Formation and Mechanistic Insight. ACS Catal. 2020, 10, 12819–12827. [Google Scholar] [CrossRef]
- Ariyananda, L.M.D.; Norman, R.E. Tris(2,2′-bipyridyl-N,N′)iron(II) bis[tetrachloroferrate(III)]. Acta Crystallogr. Sect. E 2002, 58, m775–m776. [Google Scholar] [CrossRef]
- Breu, J.; Zwicknagel, A. Chirale Erkennung bei Tris(diimin)-Metallkomplexen, 10 [1]. Vergleich der intermolekularen Wechselwirkungs- und Packungsmuster in der Reihe [Cr(bpy)3]n+(PF6)n (n = 0–3). Z. Naturforsch. B 2004, 59, 1015–1025. [Google Scholar] [CrossRef]















| REFCODE Space Group | X Y | Structure Type | Range H…Y/Å (Mean H…Y/Å) | Range C…Y/Å (Mean C…Y/Å) | Range C–H…Y/° (Mean C–H…Y/°) | Range C…X/Å (Mean C…X/Å) | Refs. |
|---|---|---|---|---|---|---|---|
| YAWWAY C2/c | S O | I | 2.182–2.442 (2.296) | 3.243–3.529 (3.370) | 163.0–175.3 (169.2) | 4.051–4.487 (4.270) | [46] |
| OLOCOI C2/c | N O | II | 2.136–2.815 (2.477) | 3.141–3.432 (3.286) | 110.8–160.6 (133.0) | 3.930–4.573 (4.158) | [47] |
| XARPEN C2/c | N O | II | 2.230–2.786 (2.491) | 3.247–3.534 (3.402) | 117.6–160.1 (143.2) | 3.836–4.532 (4.215) | [48] |
| YEJKOP C2/c | N O | II | 2.212–2.781 (2.470) | 3.234–3.518 (3.378) | 117.6–159.2 (142.8) | 3.818–4.598 (4.185) | [49] |
| REFCODE Space Group | X Y | Structure Type | Range H…Y/Å (Mean H…Y/Å) | Range C…Y/Å (Mean C…Y/Å) | Range C–H…Y/° (Mean C–H…Y/°) | Range C…X/Å (Mean C…X/Å) | Ref. |
|---|---|---|---|---|---|---|---|
| OGUNAG P21/n | N O | III | 2.369–2.813 (2.639) | 3.418–3.766 (3.602) | 127.0–174.9 (150.8) | 3.631–4.851 (4.102) | [52] |
| REFCODE Space Group | X Y | Structure Type | Range H…Y/Å (Mean H…Y/Å) | Range C…Y/Å (Mean C…Y/Å) | Range C–H…Y/° (Mean C–H…Y/°) | Range C…X/Å (Mean C…X/Å) | Refs. |
|---|---|---|---|---|---|---|---|
| XUJDEL Ccc2 | Cr O | I | 2.097–2.673 (2.347) | 3.153–3.675 (3.373) | 142.6–177.9 (160.1) | 4.106–4.737 (4.339) | [29] |
| ELIHUY C2/c | Cr O | I | 2.087–2.671 (2.319) | 3.160–3.567 (3.354) | 139.2–173.3 (161.4) | 4.080–4.723 (4.333) | [28] |
| ROLLOU C2/c | S O | II | 2.290–2.722 (2.566) | 3.170–3.780 (3.464) | 127.6–163.8 (141.0) | 3.965–4.400 (4.227) | [56] |
| MOSHUZ C2/c | S O | II | 2.287–2.786 (2.540) | 3.087–3.701 (3.388) | 116.2–158.5 (135.9) | 3.860–4.430 (4.189) | [57] |
| KOKDUM C2/c | S O | II | 2.323–2.736 (2.539) | 3.082–3.679 (3.371) | 115.9–157.9 (134.4) | 3.791–4.421 (4.146) | [58] |
| BPYNIS a C2/c | S O | II | 2.261–2.658 (2.518) | 3.106–3.716 (3.407) | 125.8–163.8 (140.2) | 3.896–4.385 (4.189) | [59] |
| OREHEZ C2/c | S O | II | 2.308–2.769 (2.551) | 3.090–3.696 (3.388) | 116.3–157.3 (134.8) | 3.816–4.416 (4.157) | [60] |
| LIFDOV C2/c | S O | II | 2.300–2.741 (2.554) | 3.160–3.633 (3.445) | 124.8–163.9 (140.6) | 3.965–4.397 (4.217) | [61] |
| KOLXOY C2/c | S O/S | II | Y = S 2.814–2.999 (2.901) Y = O 2.299–2.763 (2.556) | Y = S 3.735–3.895 (3.821) Y = O 3.187–3.756 (3.490) | Y = S 131.1–156.1 (143.5) Y = O 124.4–166.2 (145.4) | 4.113–4.595 (4.323) | [62] |
| ROKHII C2/c | S O/S | II | Y = S 2.774–3.010 (2.891) Y = O 2.320–2.785 (2.563) | Y = S 3.711–3.866 (3.798) Y = O 3.161–3.850 (3.502) | Y = S 127.7–155.7 (142.3) Y = O 124.3–167.4 (146.1) | 4.090–4.637 (4.319) | [63] |
| RUYYIU b C2/c | Cr O | II | 2.257–2.778 (2.576) | 3.185–3.685 (3.381) | 113.9–169.9 (132.7) | 4.016–4.697 (4.313) | [64] |
| NEMPAW b C2/c | Cr O | II | 2.198–2.816 (2.602) | 3.197–3.779 (3.408) | 115.0–172.1 (133.1) | 4.078–4.700 (4.333) | [65] |
| VADPUM b C2/c | Cr O | II | 2.147–2.789 (2.587) | 3.203–3.732 (3.394) | 116.8–166.1 (133.0) | 4.034–4.691 (4.327) | [66] |
| ELIMUH a C2/c | Mo O | II | 2.297–2.804 (2.588) | 3.120–3.882 (3.419) | 110.4–170.3 (135.6) | 3.982–5.100 (4.350) | [67] |
| REFCODE Space Group | X Y | Structure Type | Range H…Y/Å (Mean H…Y/Å) | Range C…Y/Å (Mean C…Y/Å) | Range C–H…Y/° (Mean C–H…Y/°) | Range C…X/Å (Mean C…X/Å) | Refs. |
|---|---|---|---|---|---|---|---|
| RAHRUO a C2221 | Cl O | II | 2.010–2.805 (2.569) | 2.979–3.750 (3.419) | 107.5–169.8 (137.8) | 3.870–4.878 (4.369) | [69] |
| YUMJIZ a C2 | Cl O | II | 2.12–2.79 (2.52) | 3.04–3.66 (3.31) | 111.8–150.4 (130.1) | 4.07–4.55 (4.27) | [71] |
| YUMJOF a C2 | Cl O | II | 2.19–2.82 (2.53) | 3.07–3.61 (3.32) | 107.0–148.4 (130.4) | 4.05–4.64 (4.26) | [71] |
| REFCODE Space Group | X Y | Structure Type | Range H…Y/Å (Mean H…Y/Å) | Range C…Y/Å (Mean C…Y/Å) | Range C–H…Y/° (Mean C–H…Y/°) | Range C…X/Å (Mean C…X/Å) | Refs. |
|---|---|---|---|---|---|---|---|
| MEFCEG C2/c | Cl O | IV | 2.575–2.779 (2.688) | 3.226–3.716 (3.419) | 110.8–146.5 (125.2) | 3.801–4.717 (4.304) | [76] |
| WADBOT C2/c | Cl O | IV | 2.534–2.807 (2.692) | 3.200–3.713 (3.417) | 114.0–147.5 (124.7) | 3.810–4.672 (4.279) | [78] |
| WADBOT01 C2/c | Cl O | IV | 2.513–2.808 (2.694) | 3.182–3.682 (3.409) | 109.4–147.3 (123.8) | 3.796–4.666 (4.252) | [79] |
| WADBOT02 C2/c | Cl O | IV | 2.512–2.754 (2.625) | 3.150–3.595 (3.335) | 109.0–147.4 (123.3) | 3.677–4.578 (4.183) | [80] |
| HEGMIP C2/c | Cl O | IV | 2.411–2.794 (2.698) | 3.151–3.706 (3.417) | 105.9–151.1 (124.6) | 3.873–4.668 (4.279) | [72] |
| HEGMIP01 C2/c | Cl O | IV | 2.466–2.754 (2.688) | 3.141–3.691 (3.409) | 109.5–150.3 (124.6) | 3.873–4.664 (4.273) | [71] |
| HEGMIP02 C2/c | Cl O | IV | 2.526–2.726 (2.642) | 3.140–3.698 (3.369) | 109.1–147.8 (124.9) | 3.732–4.565 (4.148) | [73] |
| ZAMGAV C2/c | Cl O | IV | 2.522–2.790 (2.693) | 3.216–3.521 (3.390) | 107.4–145.6 (123.9) | 3.776–4.593 (4.201) | [75] |
| ZAMGAV01 a C2/c | Cl O | IV | 2.541–2.738 (2.642) | 3.183–3.680 (3.372) | 110.0–147.3 (125.1) | 3.760–4.695 (4.263) | [74] |
| ZAMGAV02 C2/c | Cl O | IV | 2.581–2.774 (2.675) | 3.213–3.712 (3.405) | 111.2–145.1 (125.1) | 3.746–4.737 (4.285) | [71] |
| WOBTIQ C2/c | Cl O | IV | 2.507–2.812 (2.664) | 3.084–3.603 (3.366) | 107.4–149.5 (122.8) | 3.749–4.541 (4.207) | [77] |
| REFCODE Space Group | X Y | Structure Type | Range H…Y/Å (Mean H…Y/Å) | Range C…Y/Å (Mean C…Y/Å) | Range C–H…Y/° (Mean C–H…Y/°) | Range C…X/Å (Mean C…X/Å) | Refs. |
|---|---|---|---|---|---|---|---|
| TEXDAC P21 | Pd Cl | I | 2.582–3.030 (2.809) | 3.460–3.980 (3.642) | 117.6–176.5 (147.6) | 3.762–5.770 (4.733) | [81] |
| BINZON c | Cd Cl | II | 2.609–3.037 (2.827) | 3.490–3.856 (3.713) | 127.0–156.2 (141.9) | 4.41–5.04 (4.65) | [82] |
| XEQCUR a c | Zn Cl | II | 2.588–2.841 b (2.715) | 3.508–3.861 (3.685) | 141.6–155.8 (148.7) | 4.390–4.458 (4.424) | [83] |
| NALSUQ c | Fe Cl | II | 2.551–2.816 (2.683) | 3.472–3.840 (3.656) | 141.7–156.6 (149.1) | 4.387–4.455 (4.421) | [84] |
| SABGOV P6322 | Mn Br | II | 2.863–2.979 (2.950) | 3.802–4.012 (3.887) | 136.3–158.6 (146.0) | 4.335–4.730 (4.483) | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constable, E.C.; Housecroft, C.E. Embracing [XY3]m– and [XY4]m– Anions in Salts of [M(bpy)3]q+. Crystals 2023, 13, 97. https://doi.org/10.3390/cryst13010097
Constable EC, Housecroft CE. Embracing [XY3]m– and [XY4]m– Anions in Salts of [M(bpy)3]q+. Crystals. 2023; 13(1):97. https://doi.org/10.3390/cryst13010097
Chicago/Turabian StyleConstable, Edwin C., and Catherine E. Housecroft. 2023. "Embracing [XY3]m– and [XY4]m– Anions in Salts of [M(bpy)3]q+" Crystals 13, no. 1: 97. https://doi.org/10.3390/cryst13010097
APA StyleConstable, E. C., & Housecroft, C. E. (2023). Embracing [XY3]m– and [XY4]m– Anions in Salts of [M(bpy)3]q+. Crystals, 13(1), 97. https://doi.org/10.3390/cryst13010097