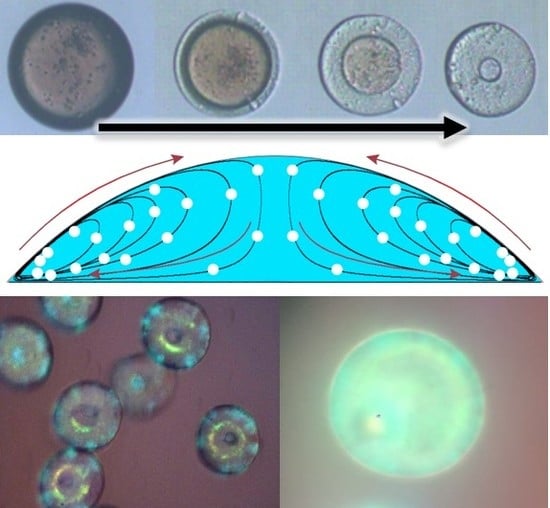
Self-Assembly Kinetics of Colloidal Particles inside Monodispersed Micro-Droplet and Fabrication of Anisotropic Photonic Crystal Micro-Particles
Abstract
:1. Introduction
2. Extraction Strength’s Effect on Macro-Structure of Photonic Crystal Particles
3. Results: Self-Assembly Kinetics of Colloidal Nanoparticle in Fabricating Anisotropic Colloidal Photonic Crystal Particles
4. Results: Optical Characterization
5. Polystyrene Photonic Crystal Particles
6. Experimental Details
6.1. Chemicals, Materials and Characterization
6.2. Colloidal Photonic Crystal Droplets Preparation
7. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yablonovitch, E. Inhibited spontaneous emission in solid-state physics and electronics. Phys. Rev. Lett. 1987, 58, 2059–2062. [Google Scholar] [CrossRef] [PubMed]
- John, S. Strong localization of photons in certain disordered dielectric superlattices. Phys. Rev. Lett. 1987, 58, 2486–2489. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C. Materials aspects of photonic crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- Wijnhoven, J.; Vos, W.L. Preparation of photonic crystals made of air spheres in titania. Science 1998, 281, 802–804. [Google Scholar] [CrossRef]
- Vlasov, Y.A.; Bo, X.Z.; Sturm, J.C.; Norris, D.J. On-chip natural assembly of silicon photonic bandgap crystals. Nature 2001, 414, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.Y.; Yi, G.R.; Pine, D.J.; Yang, S.M. Microwave-assisted self-organization of colloidal particles in confining aqueous droplets. J. Am. Chem. Soc. 2006, 128, 10897–10904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, Z.; Yang, B. Self-assembly of photonic crystals from polymer colloids. Curr. Opin. Colloid Interface Sci. 2009, 14, 103–114. [Google Scholar] [CrossRef]
- Xu, J.H.; Ge, X.H.; Chen, R.; Luo, G.S. Microfluidic preparation and structure evolution of double emulsions with two-phase cores. RSC Adv. 2014, 4, 1900–1906. [Google Scholar] [CrossRef]
- Nie, Z.H.; Xu, S.Q.; Seo, M.; Lewis, P.C.; Kumacheva, E. Polymer particles with various shapes and morphologies produced in continuous microfluidic reactors. J. Am. Chem. Soc. 2005, 127, 8058–8063. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, J.-H.; Lu, Y.-C.; Luo, G.-S. A novel method of fabricating, adjusting, and optimizing polystyrene colloidal crystal nonspherical microparticles from gas–water janus droplets in a double coaxial microfluidic device. Cryst. Growth Des. 2014, 14, 401–405. [Google Scholar] [CrossRef]
- Nisisako, T.; Torii, T. Formation of biphasic janus droplets in a microfabricated channel for the synthesis of shape-controlled polymer microparticles. Adv. Mater. 2007, 19, 1489–1493. [Google Scholar] [CrossRef]
- Lan, W.; Li, S.; Xu, J.; Luo, G. A one-step microfluidic approach for controllable preparation of nanoparticle-coated patchy microparticles. Microfluid Nanofluid 2012, 13, 491–498. [Google Scholar] [CrossRef]
- Prasad, N.; Perumal, J.; Choi, C.-H.; Lee, C.-S.; Kim, D.-P. Generation of monodisperse inorganic–organic janus microspheres in a microfluidic device. Adv. Funct. Mater. 2009, 19, 1656–1662. [Google Scholar] [CrossRef]
- Ono, T.; Yamada, M.; Suzuki, Y.; Taniguchi, T.; Seki, M. One-step synthesis of spherical/nonspherical polymeric microparticles using non-equilibrium microfluidic droplets. RSC Adv. 2014, 4, 13557–13564. [Google Scholar] [CrossRef]
- Asher, S.A.; Holtz, J.; Liu, L.; Wu, Z.J. Self-assembly motif for creating submicron periodic materials. Polymerized crystalline colloidal arrays. J. Am. Chem. Soc. 1994, 116, 4997–4998. [Google Scholar] [CrossRef]
- Rundquist, P.A.; Photinos, P.; Jagannathan, S.; Asher, S.A. Dynamical bragg-diffraction from crystalline colloidal arrays. J. Chem. Phys. 1989, 91, 4932–4941. [Google Scholar] [CrossRef]
- Jiang, P.; Bertone, J.F.; Hwang, K.S.; Colvin, V.L. Single-crystal colloidal multilayers of controlled thickness. Chem. Mater. 1999, 11, 2132–2140. [Google Scholar] [CrossRef]
- Mihi, A.; Ocana, M.; Miguez, H. Oriented colloidal-crystal thin films by spin-coating microspheres dispersed in volatile media. Adv. Mater. 2006, 18, 2244–2249. [Google Scholar] [CrossRef]
- Jiang, P.; McFarland, M.J. Large-scale fabrication of wafer-size colloidal crystals, macroporous polymers and nanocomposites by spin-coating. J. Am. Chem. Soc. 2004, 126, 13778–13786. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Kitaev, V.; Ozin, G.A. Colloidal crystal films: Advances in universality and perfection. J. Am. Chem. Soc. 2003, 125, 15589–15598. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, X.W.; Zhao, Y.J.; Zhu, R.; Gu, Z.Z. Fabrication of colloidal crystal beads by a drop-breaking technique and their application as bioassays. Small 2008, 4, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jeon, S.-J.; Yi, G.-R.; Heo, C.-J.; Choi, J.H.; Yang, S.-M. Optofluidic assembly of colloidal photonic crystals with controlled sizes, shapes, and structures. Adv. Mater. 2008, 20, 1649–1655. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, Y.; Ito, F.; Chen, H.-H.; Nagai, K.; Zhao, Y.-H.; Gu, Z.-Z. Colloidal crystal beads as supports for biomolecular screening. Angew. Chem. Int. Ed. 2006, 45, 6835–6838. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, J.H.; Lu, Y.C.; Luo, G.S. Extraction-derived self-organization of colloidal photonic crystal particles within confining aqueous droplets. Cryst. Growth Des. 2013, 13, 926–935. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, C.-F.; Ling, L.; Chen, L.; Chen, S. Triphase microfluidic-directed self-assembly: Anisotropic colloidal photonic crystal supraparticles and multicolor patterns made easy. Angew. Chem. Int. Ed. 2012, 51, 2375–2378. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Jeon, S.-J.; Jeong, W.C.; Park, H.S.; Yang, S.-M. Optofluidic synthesis of electroresponsive photonic janus balls with isotropic structural colors. Adv. Mater. 2008, 20, 4129–4134. [Google Scholar] [CrossRef]
- Kim, S.H.; Lim, J.M.; Jeong, W.C.; Choi, D.G.; Yang, S.M. Patterned colloidal photonic domes and balls derived from viscous photocurable suspensions. Adv. Mater. 2008, 20, 3211–3217. [Google Scholar] [CrossRef]
- Kuang, M.X.; Wang, L.B.; Song, Y.L. Controllable printing droplets for high-resolution patterns. Adv. Mater. 2014, 26, 6950–6958. [Google Scholar] [CrossRef] [PubMed]
- Kuang, M.; Wu, L.; Li, Y.; Gao, M.; Zhang, X.; Jiang, L.; Song, Y. Sliding three-phase contact line of printed droplets for single-crystal arrays. Nanotechnology 2016, 27, 184002. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Dong, Z.C.; Kuang, M.X.; Li, Y.N.; Li, F.Y.; Jiang, L.; Song, Y.L. Printing patterned fine 3D structures by manipulating the three phase contact line. Adv. Funct. Mater. 2015, 25, 2237–2242. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Yunker, P.J.; Still, T.; Lohr, M.A.; Yodh, A.G. Suppression of the coffee-ring effect by shape-dependent capillary interactions. Nature 2011, 476, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Sempels, W.; De Dier, R.; Mizuno, H.; Hofkens, J.; Vermant, J. Auto-production of biosurfactants reverses the coffee ring effect in a bacterial system. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.G.; Shum, H.C.; Weitz, D.A. Fabrication of monodisperse toroidal particles by polymer solidification in microfluidics. ChemPhysChem 2009, 10, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Stober, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-Y.; Xu, K.; Xu, J.-H.; Luo, G.-S. Self-Assembly Kinetics of Colloidal Particles inside Monodispersed Micro-Droplet and Fabrication of Anisotropic Photonic Crystal Micro-Particles. Crystals 2016, 6, 122. https://doi.org/10.3390/cryst6100122
Zhang M-Y, Xu K, Xu J-H, Luo G-S. Self-Assembly Kinetics of Colloidal Particles inside Monodispersed Micro-Droplet and Fabrication of Anisotropic Photonic Crystal Micro-Particles. Crystals. 2016; 6(10):122. https://doi.org/10.3390/cryst6100122
Chicago/Turabian StyleZhang, Ming-Yu, Ke Xu, Jian-Hong Xu, and Guang-Sheng Luo. 2016. "Self-Assembly Kinetics of Colloidal Particles inside Monodispersed Micro-Droplet and Fabrication of Anisotropic Photonic Crystal Micro-Particles" Crystals 6, no. 10: 122. https://doi.org/10.3390/cryst6100122
APA StyleZhang, M. -Y., Xu, K., Xu, J. -H., & Luo, G. -S. (2016). Self-Assembly Kinetics of Colloidal Particles inside Monodispersed Micro-Droplet and Fabrication of Anisotropic Photonic Crystal Micro-Particles. Crystals, 6(10), 122. https://doi.org/10.3390/cryst6100122