ZnO Micro- and Nanostructures Obtained by Thermal Oxidation: Microstructure, Morphogenesis, Optical, and Photoluminescence Properties
Abstract
:1. Introduction
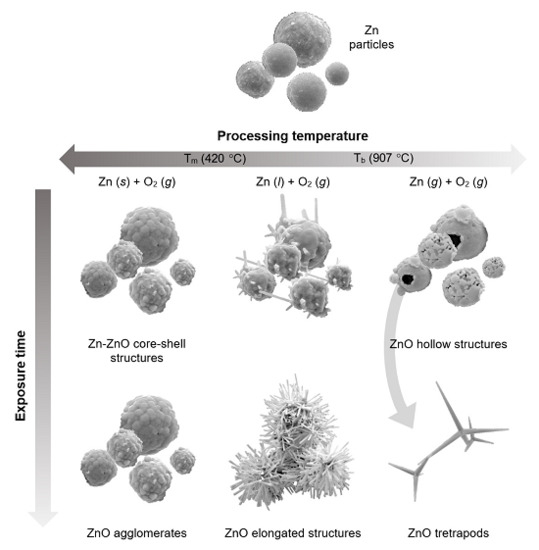
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 41301. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, C.; Cai, R.; Wang, Y.; Zhou, G. Temperature-dependent growth mechanism and microstructure of ZnO nanostructures grown from the thermal oxidation of zinc. J. Cryst. Growth 2014, 390, 101–108. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, L.; Guo, S. Insight into the Structures and Properties of Morphology-Controlled Legs of Tetrapod-Like ZnO Nanostructures. J. Phys. Chem. C 2007, 111, 12939–12943. [Google Scholar] [CrossRef]
- Zhou, Z.; Deng, H.; Yi, J.; Liu, S. A new method for preparation of zinc oxide whiskers. Mater. Res. Bull. 1999, 34, 1563–1567. [Google Scholar] [CrossRef]
- Liang, H.; Pan, L.; Liu, Z. Synthesis and photoluminescence properties of ZnO nanowires and nanorods by thermal oxidation of Zn precursors. Mater. Lett. 2008, 62, 1797–1800. [Google Scholar] [CrossRef]
- Mihailova, I.; Gerbreders, V.; Tamanis, E.; Sledevskis, E.; Viter, R.; Sarajevs, P. Synthesis of ZnO nanoneedles by thermal oxidation of Zn thin films. J. Non-Cryst. Solids 2013, 377, 212–216. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Huang, B.; Li, Q.; Zheng, Z. Using Zn as target to fabricate ZnO coating by thermal oxidation in air on quartz substrate. Opt. Commun. 2012, 285, 4290–4293. [Google Scholar] [CrossRef]
- Bae, D.; Lee, G.-H. Characterization of ZnO Tetrapods Prepared by a Simple Oxidation of Zn Plate in Air Atmosphere. Jpn. J. Appl. Phys. 2012, 51, 06FG01. [Google Scholar] [CrossRef]
- Qiu, Y.; Luo, Q.; Liang, Z.; Cheng, Z.; Guo, B.; Fan, H.; Yang, S. Preparation and characterization of ZnO tetrapods and octapods. Mater. Lett. 2015, 154, 103–106. [Google Scholar] [CrossRef]
- Tang, H.; Chang, J.C.; Shan, Y.; Ma, D.D.D.; Lui, T.-Y.; Zapien, J.A.; Lee, C.-S.; Lee, S.-T. Growth mechanism of ZnO nanowires via direct Zn evaporation. J. Mater. Sci. 2009, 44, 563–571. [Google Scholar] [CrossRef]
- Shen, G.; Bando, Y.; Lee, C.-J. Synthesis and Evolution of Novel Hollow ZnO Urchins by a Simple Thermal Evaporation Process. J. Phys. Chem. B 2005, 109, 10578–10583. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Ma, J.; Kim, Y.; Sun, Y.; Wong, G.K.L.; Ketterson, J.B. Photoluminescence and ultraviolet lasing of polycrystalline ZnO thin films prepared by the oxidation of the metallic Zn. Appl. Phys. Lett. 1999, 75, 2761–2763. [Google Scholar] [CrossRef]
- Zhang, X.T.; Liu, Y.C.; Zhang, L.G.; Zhang, J.Y.; Lu, Y.M.; Shen, D.Z.; Xu, W.; Zhong, G.Z.; Fan, X.W.; Kong, X.G. Structure and optically pumped lasing from nanocrystalline ZnO thin films prepared by thermal oxidation of ZnS thin films. J. Appl. Phys. 2002, 92, 3293–3298. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, H.; Gao, Y.; Wang, J.; Liu, D.; Shen, J.; Liu, L.; Zhou, W.; Xie, S.; Wang, X.; et al. Large-scale synthesis and optical behaviors of ZnO tetrapods. Appl. Phys. Lett. 2007, 90, 153116. [Google Scholar] [CrossRef]
- Law, J.B.K.; Thong, J.T.L. Simple fabrication of a ZnO nanowire photodetector with a fast photoresponse time. Appl. Phys. Lett. 2006, 88, 133114. [Google Scholar] [CrossRef]
- Zhao, C.X.; Li, Y.F.; Zhou, J.; Li, L.Y.; Deng, S.Z.; Xu, N.S.; Chen, J. Large-Scale Synthesis of Bicrystalline ZnO Nanowire Arrays by Thermal Oxidation of Zinc Film: Growth Mechanism and High-Performance Field Emission. Cryst. Growth Des. 2013, 13, 2897–2905. [Google Scholar] [CrossRef]
- Singh, S.; Chakrabarti, P. Comparison of the structural and optical properties of ZnO thin films deposited by three different methods for optoelectronic applications. Superlattices Microstruct. 2013, 64, 283–293. [Google Scholar] [CrossRef]
- Mishra, Y.K.; Modi, G.; Cretu, V.; Postica, V.; Lupan, O.; Reimer, T.; Paulowicz, I.; Hrkac, V.; Benecke, W.; Kienle, L.; et al. Direct Growth of Freestanding ZnO Tetrapod Networks for Multifunctional Applications in Photocatalysis, UV Photodetection, and Gas Sensing. ACS Appl. Mater. Interfaces 2015, 7, 14303–14316. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Liu, W.; Jiang, G.; Zhu, C. Study of oxygen vacancies’ influence on the lattice parameter in ZnO thin film. Mater. Lett. 2012, 85, 25–28. [Google Scholar] [CrossRef]
- Arguello, C.A.; Rousseau, D.L.; Porto, S.P.S. First-Order Raman Effect in Wurtzite-Type Crystals. Phys. Rev. 1969, 181, 1351–1363. [Google Scholar] [CrossRef]
- Calleja, J.M.; Cardona, M. Resonant Raman scattering in ZnO. Phys. Rev. B 1977, 16, 3753–3761. [Google Scholar] [CrossRef]
- Vollmer, M. Newton’s law of cooling revisited. Eur. J. Phys. 2009, 30, 1063–1084. [Google Scholar] [CrossRef]
- Wang, Y.G.; Lau, S.P.; Lee, H.W.; Yu, S.F.; Tay, B.K.; Zhang, X.H.; Hng, H.H. Photoluminescence study of ZnO films prepared by thermal oxidation of Zn metallic films in air. J. Appl. Phys. 2003, 94, 354–358. [Google Scholar] [CrossRef]
- Rusu, G.G.; Rusu, M.; Apetroaei, N. On the structural changes during thermal oxidation of evaporated Zn thin films. Thin Solid Films 2007, 515, 8699–8704. [Google Scholar] [CrossRef]
- Haynes, W.M.; Lide, D.R.; Bruno, T.J. CRC Handbook of Chemistry and Physics: A Ready–Reference Book of Chemical and Physical Data, 94th ed.; CRC Press: New York, NY, USA, 2013; pp. 4–123. [Google Scholar]
- Khanlary, M.R.; Vahedi, V.; Reyhani, A. Synthesis and Characterization of ZnO Nanowires by Thermal Oxidation of Zn Thin Films at Various Temperatures. Molecules 2012, 17, 5021–5029. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhang, Y.; Wang, Z.L. The octa-twin tetraleg ZnO nanostructures. Solid State Commun. 2003, 126, 629–633. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Kang, Z.; Bello, I.; Fan, X.; Shafiq, I.; Zhang, W.; Lee, S.-T. Self-catalytic Synthesis of ZnO Tetrapods, Nanotetraspikes, and Nanowires in Air at Atmospheric Pressure. J. Phys. Chem. C 2008, 112, 9214–9218. [Google Scholar] [CrossRef]
- Gui, Y.; Xie, C.; Zhang, Q.; Hu, M.; Yu, J.; Weng, Z. Synthesis and characterization of ZnO nanostructures by two-step oxidation of Zn nano- and microparticles. J. Cryst. Growth 2006, 289, 663–669. [Google Scholar] [CrossRef]
- Modi, G. Zinc oxide tetrapod: A morphology with multifunctional applications. Adv. Nat. Sci. Nanosci. Nanotechnol. 2015, 6, 33002. [Google Scholar] [CrossRef]
- Ma, L.A.; Guo, T.L. Morphology control and improved field emission properties of ZnO tetrapod films deposited by electrophoretic deposition. Ceram. Inter. 2013, 39, 6923–6929. [Google Scholar] [CrossRef]
- Wan, Q.; Yu, K.; Wang, T.H.; Lin, C.L. Low-field electron emission from tetrapod-like ZnO nanostructures synthesized by rapid evaporation. Appl. Phys. Lett. 2003, 83, 2253–2255. [Google Scholar] [CrossRef]
- Wang, B.-B.; Xie, J.-J.; Yuan, Q.; Zhao, Y.-P. Growth mechanism and joint structure of ZnO tetrapods. J. Phys. D Appl. Phys. 2008, 41, 102005. [Google Scholar] [CrossRef]
- Lee, G.-H. Synthesis and cathodoluminescence of ZnO tetrapods prepared by a simple oxidation of Zn powder in air atmosphere. Ceram. Inter. 2011, 37, 189–193. [Google Scholar] [CrossRef]
- Du, G.-P.; Li, W.; Fu, M.-G.; Chen, N.; Fu, X.; Wan, Y.-Q.; Yan, M.-M. Synthesis of tetrapod-shaped ZnO whiskers and microrods in one crucible by thermal evaporation of Zn/C mixtures. Trans. Nonferr. Metal Soc. Chin. 2008, 18, 155–161. [Google Scholar] [CrossRef]
- Newton, M.C.; Warburton, P.A. ZnO tetrapod nanocrystals. Mater. Today 2007, 10, 50–54. [Google Scholar] [CrossRef]
- Fuller, M.L. Twinning in Zinc Oxide. J. Appl. Phys. 1944, 15, 164–170. [Google Scholar] [CrossRef]
- Shiojiri, M.; Kaito, C. Structure and growth of ZnO smoke particles prepared by gas evaporation technique. J. Cryst. Growth 1981, 52, 173–177. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Z.L.; Sun, T.; Qiu, J. Zinc-blende ZnO and its role in nucleating wurtzite tetrapods and twinned nanowires. Appl. Phys. Lett. 2007, 90, 153510. [Google Scholar] [CrossRef]
- Kitano, M.; Hamabe, T.; Maeda, S.; Okabe, T. Growth of large tetrapod-like ZnO crystals II. Morphological considerations on growth mechanism. J. Cryst. Growth 1991, 108, 277–284. [Google Scholar] [CrossRef]
- Nishio, K.; Isshiki, T.; Kitano, M.; Shiojiri, M. Structure and growth mechanism of tetrapod-like ZnO particles. Philos. Mag. A 1997, 76, 889–904. [Google Scholar] [CrossRef]
- Iwanaga, H.; Fujii, M.; Takeuchi, S. Growth model of tetrapod zinc oxide particles. J. Cryst. Growth 1993, 134, 275–280. [Google Scholar] [CrossRef]
- Takeuchi, S.; Iwanaga, H.; Fujii, M. Octahedral multiple-twin model of tetrapod ZnO crystals. Philos. Mag. A 1994, 69, 1125–1129. [Google Scholar] [CrossRef]
- Iwanaga, H.; Fujii, M.; Takeuchi, S. Inter-leg angles in tetrapod ZnO particles. J. Cryst. Growth 1998, 183, 190–195. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.-M.; Zhang, Y.-M.; Lü, H.-L. Interfacial characteristics of Al/Al2O3/ZnO/n-GaAs MOS capacitor. Chinese Phys. B 2013, 22, 76701. [Google Scholar] [CrossRef]
- Wang, F.; Ye, Z.; Ma, D.; Zhu, L.; Zhuge, F. Rapid synthesis and photoluminescence of novel ZnO nanotetrapods. J. Cryst. Growth 2005, 274, 447–452. [Google Scholar] [CrossRef]
- Lyapina, O.A.; Baranov, A.N.; Panin, G.N.; Knotko, A.V.; Kononenko, O.V. Synthesis of ZnO nanotetrapods. Inorg. Mater. 2008, 44, 846–852. [Google Scholar] [CrossRef]
- Yan, H.; He, R.; Pham, J.; Yang, P. Morphogenesis of One-Dimensional ZnO Nano-and Microcrystals. Adv. Mater. 2003, 15, 402–405. [Google Scholar] [CrossRef]
- Gorobchenko, V.D.; Zharnikov, M.V.; Maksimov, E.G.; Rashkeev, S.N. Experimental and theoretical investigations of plasma oscillations of electrons in zinc and cadmium. Sov. Phys. JETP 1985, 61, 398–406. [Google Scholar]
- Iribarren, A.; Castro-Rodríguez, R.; Sosa, V.; Pena, J.L. Band-tail parameter modeling in semiconductor materials. Phys. Rev. B 1998, 58, 1907–1911. [Google Scholar] [CrossRef]
- Halperin, B.I.; Lax, M. Impurity-Band Tails in the High-Density Limit. I. Minimum Counting Methods. Phys. Rev. 1966, 148, 722–740. [Google Scholar] [CrossRef]
- Janotti, A.; Van de Walle, C.G. Native point defects in ZnO. Phys. Rev. B 2007, 76, 165202. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Sánchez-Mora, E.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fis. S 2007, 53, 18–22. [Google Scholar]
- Rackauskas, S.; Klimova, O.; Jiang, H.; Nikitenko, A.; Chernenko, K.A.; Shandakov, S.D.; Kauppinen, E.I.; Tolochko, O.V.; Nasibulin, A.G. A Novel Method for Continuous Synthesis of ZnO Tetrapods. J. Phys. Chem. C 2015, 119, 16366–16373. [Google Scholar] [CrossRef]
- Rodnyi, P.A.; Khodyuk, I.V. Optical and luminescence properties of zinc oxide (Review). Opt. Spectrosc. 2011, 111, 776–785. [Google Scholar] [CrossRef]
- Yang, L.L.; Zhao, Q.X.; Willander, M.; Yang, J.H.; Ivanov, I. Annealing effects on optical properties of low temperature grown ZnO nanorod arrays. J. Appl. Phys. 2009, 105, 53503. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Correlation between photoluminescence and oxygen vacancies in ZnO phosphors. Appl. Phys. Lett. 1996, 68, 403–405. [Google Scholar] [CrossRef]
- Wang, Y.W.; Zhang, L.D.; Wang, G.Z.; Peng, X.S.; Chu, Z.Q.; Liang, C.H. Catalytic growth of semiconducting zinc oxide nanowires and their photoluminescence properties. J. Cryst. Growth 2002, 234, 171–175. [Google Scholar] [CrossRef]
- Studenikin, S.A.; Golego, N.; Cocivera, M. Fabrication of green and orange photoluminescent, undoped ZnO films using spray pyrolysis. J. Appl. Phys. 1998, 84, 2287–2294. [Google Scholar] [CrossRef]
- Wu, X.L.; Siu, G.G.; Fu, C.L.; Ong, H.C. Photoluminescence and cathodoluminescence studies of stoichiometric and oxygen-deficient ZnO films. Appl. Phys. Lett. 2001, 78, 2285–2287. [Google Scholar] [CrossRef]
- Zeng, H.-B.; Duan, G.-T.; Li, Y.; Yang, S.-K.; Xu, X.-X.; Cai, W.-P. Blue Luminescence of ZnO Nanoparticles Based on Non-Equilibrium Processes: Defect Origins and Emission Controls. Adv. Funct. Mater. 2010, 20, 561–572. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Ntwaeaborwa, O.M.; Kroon, R.E.; Terblans, J.J.; Shaat, S.K.K.; Yousif, A.; Duvenhage, M.M. Origin of the red emission in zinc oxide nanophosphors. Mater. Lett. 2013, 101, 57–60. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Leung, Y.H.; Tam, K.H.; Ding, L.; Ge, W.K.; Chen, H.Y.; Gwo, S. Green, yellow, and orange defect emission from ZnO nanostructures: Influence of excitation wavelength. Appl. Phys. Lett. 2006, 88, 103107. [Google Scholar] [CrossRef]







| S0 (0 min) | S1 (3 min) | S2 (6 min) | S3 (12 min) | S4 (24 min) | h-Zn | w-ZnO |
|---|---|---|---|---|---|---|
| 2θ; FWHM; d (°; °; Å) | hkl; d (Å) | |||||
| 31.85; 0.23; 2.810 * | 31.79; 0.10; 2.812 | 31.79; 0.08; 2.813 | 31.74; 0.09; 2.817 | 31.75; 0.16; 2.818 * | --- | 100; 2.814 |
| 34.46; 0.20; 2.602 * | 34.45; 0.10; 2.601 | 34.45; 0.08; 2.601 | 34.39; 0.09; 2.605 | 34.41; 0.16; 2.606 * | --- | 002; 2.603 |
| 36.29; 0.08; 2.473 | 36.29; 0.07; 2.474 | 36.29; 0.07; 2.474 | 36.22; 0.09; 2.478 | 36.24; 0.17; 2.479 * | 002; 2.473 | 101; 2.476 |
| 39.00; 0.07; 2.308 | 38.99; 0.09; 2.308 | 39.03; 0.05; 2.306 | --- | --- | 100; 2.308 | --- |
| 43.21; 0.09; 2.092 | 43.24; 0.06; 2.091 | 43.25; 0.07; 2.090 | --- | --- | 101; 2.091 | --- |
| 47.60; 0.28; 1.911 * | 47.56; 0.08; 1.910 | 47.57; 0.09; 1.910 | 47.50; 0.09; 1.912 | 47.51; 0.13; 1.912 | --- | 102; 1.911 |
| 54.33; 0.07; 1.687 | 54.33; 0.08; 1.687 | 54.35; 0.08; 1.687 | --- | --- | 102; 1.687 | --- |
| 56.69; 0.37; 1.624 * | 56.61; 0.09; 1.624 | 56.62; 0.10; 1.624 | 56.56; 0.10; 1.626 | 56.56; 0.14; 1.626 | --- | 110; 1.625 |
| 62.91; 0.36; 1.477 * | 62.88; 0.12; 1.477 | 62.88; 0.10; 1.477 | 62.83; 0.11; 1.478 | 62.83; 0.14; 1.478 | --- | 103; 1.477 |
| 66.55; 0.61; 1.405 * | 66.40; 0.12; 1.407 | 66.40; 0.10; 1.407 | 66.34; 0.12; 1.408 | 66.33; 0.15; 1.408 | --- | 200; 1.407 |
| 68.05; 0.47; 1.378 * | 67.97; 0.12; 1.378 | 67.98; 0.11; 1.378 | 67.91; 0.11; 1.379 | 67.91; 0.14; 1.379 | --- | 112; 1.378 |
| 69.23; 0.56; 1.357 * | 69.11; 0.13; 1.358 | 69.12; 0.14; 1.358 | 69.05; 0.11; 1.359 | 69.04; 0.14; 1.359 | --- | 201; 1.358 |
| 70.06; 0.09; 1.342 | 70.06; 0.09; 1.342 | 70.08; 0.09; 1.342 | --- | --- | 103; 1.342 | --- |
| 70.65; 0.08; 1.332 | 70.66; 0.07; 1.332 | 70.66; 0.07; 1.332 | --- | --- | 110; 1.332 | --- |
| --- | --- | 72.60; 0.13; 1.301 | 72.53; 0.10; 1.302 | 72.53; 0.13; 1.302 | --- | 004; 1.302 |
| 77.01; 0.12; 1.237 | 77.03; 0.09; 1.237 | 77.03; 0.14; 1.237 | 76.92; 0.12; 1.239 | 76.92; 0.15; 1.238 | --- | 202; 1.238 |
| Sample | Zn:O (avg. at.%) | IUV/IVis (a.u.) | ZnO–Eg (eV) |
|---|---|---|---|
| S0 | 81.4:18.6 | 2.49 | 3.23 ± 0.05 |
| S1 | 66.3:33.7 | 0.64 | 3.22 ± 0.04 |
| S2 | 65.2:34.8 | 0.49 | 3.22 ± 0.04 |
| S3 | 60.4:39.6 | 0.06 | 3.21 ± 0.03 |
| S4 | 58.7:41.3 | 0.11 | 3.22 ± 0.02 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escobedo-Morales, A.; Aranda-García, R.J.; Chigo-Anota, E.; Pérez-Centeno, A.; Méndez-Blas, A.; Arana-Toro, C.G. ZnO Micro- and Nanostructures Obtained by Thermal Oxidation: Microstructure, Morphogenesis, Optical, and Photoluminescence Properties. Crystals 2016, 6, 135. https://doi.org/10.3390/cryst6100135
Escobedo-Morales A, Aranda-García RJ, Chigo-Anota E, Pérez-Centeno A, Méndez-Blas A, Arana-Toro CG. ZnO Micro- and Nanostructures Obtained by Thermal Oxidation: Microstructure, Morphogenesis, Optical, and Photoluminescence Properties. Crystals. 2016; 6(10):135. https://doi.org/10.3390/cryst6100135
Chicago/Turabian StyleEscobedo-Morales, Alejandro, Rubén J. Aranda-García, Ernesto Chigo-Anota, Armando Pérez-Centeno, Antonio Méndez-Blas, and Carlos G. Arana-Toro. 2016. "ZnO Micro- and Nanostructures Obtained by Thermal Oxidation: Microstructure, Morphogenesis, Optical, and Photoluminescence Properties" Crystals 6, no. 10: 135. https://doi.org/10.3390/cryst6100135
APA StyleEscobedo-Morales, A., Aranda-García, R. J., Chigo-Anota, E., Pérez-Centeno, A., Méndez-Blas, A., & Arana-Toro, C. G. (2016). ZnO Micro- and Nanostructures Obtained by Thermal Oxidation: Microstructure, Morphogenesis, Optical, and Photoluminescence Properties. Crystals, 6(10), 135. https://doi.org/10.3390/cryst6100135