E’’ Raman Mode in Thermal Strain-Fractured CVD-MoS2
Abstract
:1. Introduction
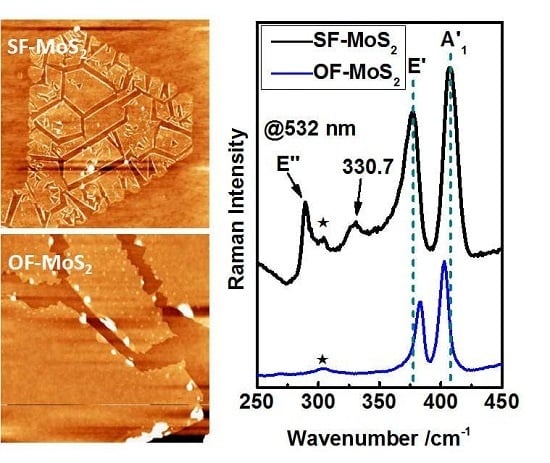
2. Result and Discussion
3. Experimental Details
3.1 Sample preparation
3.2 Characterizations of As-Grown Three kinds of MoS2 Sample
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Kufer, D.; Konstantatos, G. Highly Sensitive, Encapsulated MoS2 Photodetector with Gate Controllable Gain and Speed. Nano Lett. 2015, 15, 7307–7313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.H.; Kalantar-Zadeh, K.; Kis, A.; Coleman, J.N.; Strano, M.S. Electronics and Optoelectronics of Two-dimensional Transition Metal Dichalcogenides. Nat. Nanotechnol. 2012, 7, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Zhang, X.Q.; Zhang, W.J.; Chang, M.T.; Lin, C.T.; Chang, K.D.; Yu, Y.C.; Wang, J.T.W.; Chang, C.S.; Li, L.J.; et al. Synthesis of Large-Area MoS2 Atomic Layers with Chemical Vapor Deposition. Adv. Mater. 2012, 24, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, B.; Radenovic, A.; Brivio, J.; Giacometti, V.; Kis, A. Single-Layer MoS2 Transistors. Nat. Nanotechnol. 2011, 6, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sanchez, O.; Lembke, D.; Kayci, M.; Radenovic, A.; Kis, A. Ultrasensitive Photodetectors Based on Monolayer MoS2. Nat. Nanotecnol. 2013, 8, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Yomogida, Y.; Liu, K.K.; Li, L.J.; Iwasa, Y.; Takenobu, T. Highly Flexible MoS2 Thin-Film Transistors with Ion Gel Dielectrics. Nano Lett. 2012, 12, 4013–4017. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Xiao, S.; Li, X.H.; Lyu, B.S.; Huang, Y.B.; Cheng, S.B.; Huang, H.; He, J.; Gao, Y.L. Investigation of the Dynamic Bending Properties of MoS2 Thin Films by Interference Colours. Sci. Rep. 2015, 5, 18441. [Google Scholar] [CrossRef] [PubMed]
- Bertolazzi, S.; Brivio, J.; Kis, A. Stretching and Breaking of Ultrathin MoS2. ACS Nano 2011, 5, 9703–9709. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Gomez, A.; Poot, M.; Steele, G.A.; van der Zant, H.S.; Agrait, N.; Rubio-Bollinger, G. Elastic Properties of Freely Suspended MoS2 Nanosheets. Adv. Mater. 2012, 24, 772–775. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.Q.; Zhang, Y.; Zhang, Y.F.; Liu, Z.F. Chemical Vapour Deposition of Group-VIB Metal Dichalcogenide Monolayers: Engineered Substrates from Amorphous to Single Crystalline. Chem. Soc. Rev. 2015, 44, 2587–2602. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.S.; Sun, D.Z.; Ouyang, B.; Raja, A.; Song, J.; Heinz, T.F.; Brus, L.E. Probing the Dynamics of the Metallic-to-Semiconducting Structural Phase Transformation in MoS2 Crystals. Nano Lett. 2015, 15, 5081–5088. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.M.; Gong, Y.J.; Hu, Z.J.; Li, Z.W.; Qiu, Z.W.; Zhu, X.; Ajayan, P.M.; Fang, Z.Y. Plasmonic Hot Electron Enhanced MoS2 Photocatalysis in Hydrogen Evolution. Nanoscale 2015, 7, 4482–4488. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, S.J.; Yang, D.; Frindt, R.F.; Irwin, J.C. Raman Study and Lattice Dynamics of Single Molecular Layers of MoS2. Phys. Rev. B 1991, 44, 3955–3962. [Google Scholar] [CrossRef]
- Chen, X.B.; Chen, Z.L.; Li, J. Critical Electronic Structures Controlling Phase Transitions Induced by Lithium Ion Intercalation in Molybdenum Disulphide. Chin. Sci. Bull. 2013, 58, 1632–1641. [Google Scholar] [CrossRef]
- Kong, D.S.; Wang, H.T.; Cha, J.J.; Pasta, M.; Koski, K.J.; Yao, J.; Cui, Y. Synthesis of MoS2 and MoSe2 Films with Vertically Aligned Layers. Nano Lett. 2013, 13, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Shen, J.; Liu, Y.H.; Woods, J.M.; Sun, Y.; Cha, J.J. Metal Seed Layer Thickness-Induced Transition From Vertical to Horizontal Growth of MoS2 and WS2. Nano Lett. 2014, 14, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Yang, B.C.; Gao, Y.L. Quenching Induced Fracture Behaviors of CVD-grown Polycrystalline Molybdenum Disulfide Films. RSC Adv. 2016, 6, 59816–59822. [Google Scholar] [CrossRef]
- Kiriya, D.; Lobaccaro, P.; Nyein, H.Y.Y.; Taheri, P.; Hettick, M.; Shiraki, H.; Sutter-Fella, C.M.; Zhao, P.D.; Gao, W.; Maboudian, R.; et al. General Thermal Texturization Process of MoS2 for Efficient Electrocatalytic Hydrogen Evolution Reaction. Nano Lett. 2016, 16, 4047–4053. [Google Scholar] [CrossRef] [PubMed]
- Scheuschner, N.; Gillen, R.; Staiger, M.; Maultzsch, J. Interlayer Resonant Raman Modes in Few-Layer MoS2. Phys. Rev. B 2015, 91, 235409. [Google Scholar] [CrossRef]
- Liu, Z.; Amani, M.; Najmaei, S.; Xu, Q.; Zou, X.L.; Zhou, W.; Yu, T.; Qiu, C.Y.; Birdwell, A.G.; Crowne, F.J.; et al. Strain and Structure Heterogeneity in MoS2 Atomic Layers Grown by Chemical Vapour Deposition. Nat. Commun. 2014, 5, 5246. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.M.; He, K.; Pacios, M.; Robertson, A.W.; Bhaskaran, H.; Warner, J.H. Controlled Preferential Oxidation of Grain Boundaries in Monolayer Tungsten Disulfide for Direct Optical Imaging. ACS Nano 2015, 9, 3695–3703. [Google Scholar] [CrossRef] [PubMed]
- Nan, H.Y.; Wang, Z.L.; Wang, W.H.; Liang, Z.; Lu, Y.; Chen, Q.; He, D.W.; Tan, P.H.; Miao, F.; Wang, X.R.; et al. Strong Photoluminescence Enhancement of MoS2 through Defect Engineering and Oxygen Bonding. ACS Nano 2014, 8, 5738–5745. [Google Scholar] [CrossRef] [PubMed]
- Splendiani, A.; Sun, L.; Zhang, Y.B.; Li, T.S.; Kim, J.; Chim, C.Y.; Galli, G.; Wang, F. Emerging Photoluminescence in Monolayer MoS2. Nano Lett. 2010, 10, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yan, H.; Brus, L.E.; Heinz, T.F.; Hone, J.; Ryu, S. Anomalous Lattice Vibrations of Single- and Few-Layer MoS2. ACS Nano 2010, 4, 2695–2700. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically Thin MoS2: A New Direct-Gap Semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [PubMed]
- Li, F.F.; Yan, Y.L.; Han, B.; Li, L.; Huang, X.L.; Yao, M.G.; Gong, Y.B.; Jin, X.L.; Liu, B.L.; Zhu, C.R.; et al. Pressure Confinement Effect in MoS2 Monolayers. Nanoscale 2015, 7, 9075–9082. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.P.; Pandey, T.; Voiry, D.; Liu, J.; Moran, S.T.; Sharma, A.; Tan, C.; Chen, C.H.; Li, L.J.; Chhowalla, M.; et al. Pressure-Dependent Optical and Vibrational Properties of Monolayer Molybdenum Disulfide. Nano Lett. 2015, 15, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.M.; Ding, K.; Jiang, D.S.; Sun, B.Q. Tuning and Identification of Interband Transitions in Monolayer and Bilayer Molybdenum Disulfide Using Hydrostatic Pressure. ACS Nano 2014, 8, 7458–7464. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Yu, G.N.; Zhao, Y.W.; Xu, Q.; Xing, G.C.; Liu, X.F.; Fu, D.Y.; Liu, B.; Tan, J.R.S.; Tang, W.; et al. Achieving Ultrafast Hole Transfer at the Monolayer MoS2 and CH3NH3PbI3 Perovskite Interface by Defect Engineering. ACS Nano 2016, 10, 6383–6391. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Rong, Y.M.; Fan, Y.; Pacios, M.; Bhaskaran, H.; He, K.; Warner, J.H. Shape Evolution of Monolayer MoS2 Crystals Grown by Chemical Vapor Deposition. Chem. Mater. 2014, 26, 6371–6379. [Google Scholar] [CrossRef]
- Mignuzzi, S.; Pollard, A.J.; Bonini, N.; Brennan, B.; Gilmore, I.S.; Pimenta, M.A.; Richards, D.; Roy, D. Effect of Disorder on Raman Scattering of Single-Layer MoS2. Phys. Rev. B 2015, 91, 195411. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Yap, C.C.R.; Tay, B.K.; Edwin, T.H.T.; Olivier, A.; Baillargeat, D. From Bulk to Monolayer MoS2: Evolution of Raman Scattering. Adv. Funct. Mater. 2012, 22, 1385–1390. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- Blanc, N.; Jean, F.; Krasheninnikov, A.V.; Renaud, G.; Coraux, J. Strains Induced by Point Defects in Graphene on a Metal. Phys. Rev. Lett. 2013, 111, 085501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, B.; Bera, A.; Muthu, D.V.S.; Bhowmick, S.; Waghmare, U.V.; Sood, A.K. Symmetry-Dependent Phonon Renormalization in Monolayer MoS2 Transistor. Phys. Rev. B 2012, 85, 161403. [Google Scholar] [CrossRef]
- Parkin, W.M.; Balan, A.; Liang, L.B.; Das, P.M.; Lamparski, M.; Naylor, C.H.; Rodriguez-Manzo, J.A.; Johnson, A.T.C.; Meunier, V.; Drndic, M. Raman Shifts in Electron-Irradiated Monolayer MoS2. ACS Nano 2016, 10, 4134–4142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zou, X.L.; Najmaei, S.; Liu, Z.; Shi, Y.M.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.C. Intrinsic Structural Defects in Monolayer Molybdenum Disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef] [PubMed]





© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Huang, H.; Zhu, X.; He, Y.; Xie, Q.; Chen, X.; Zheng, X.; Duan, H.; Gao, Y. E’’ Raman Mode in Thermal Strain-Fractured CVD-MoS2. Crystals 2016, 6, 151. https://doi.org/10.3390/cryst6110151
Wu D, Huang H, Zhu X, He Y, Xie Q, Chen X, Zheng X, Duan H, Gao Y. E’’ Raman Mode in Thermal Strain-Fractured CVD-MoS2. Crystals. 2016; 6(11):151. https://doi.org/10.3390/cryst6110151
Chicago/Turabian StyleWu, Di, Han Huang, Xupeng Zhu, Yanwei He, Qiliang Xie, Xiaoliu Chen, Xiaoming Zheng, Huigao Duan, and Yongli Gao. 2016. "E’’ Raman Mode in Thermal Strain-Fractured CVD-MoS2" Crystals 6, no. 11: 151. https://doi.org/10.3390/cryst6110151
APA StyleWu, D., Huang, H., Zhu, X., He, Y., Xie, Q., Chen, X., Zheng, X., Duan, H., & Gao, Y. (2016). E’’ Raman Mode in Thermal Strain-Fractured CVD-MoS2. Crystals, 6(11), 151. https://doi.org/10.3390/cryst6110151