Synthesis, Structures and Properties of Cobalt Thiocyanate Coordination Compounds with 4-(hydroxymethyl)pyridine as Co-ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Aspects
2.2. Crystal Structures
2.2.1. Co(NCS)2(4-(hydroxymethyl)pyridine)4 (1) and Co(NCS)2(4-(hydroxymethyl)pyridine)4 × H2O (1-H2O)
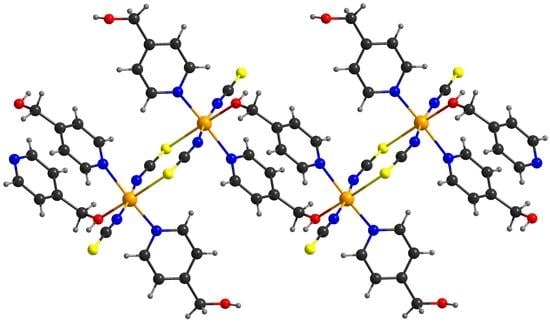
2.2.2. Co(NCS)2(4-(hydroxymethyl)pyridine)2(EtOH)2 (2) and Co(NCS)2(4-(hydroxymethyl)pyridine)2(H2O)2 (3)
2.2.3. [Co(NCS)2(4-(hydroxymethyl)pyridine)2]n∙4 H2O (4)
2.2.4. [Co(NCS)2(4-(hydroxymethyl)pyridine)2]n (5)
2.3. IR Spectroscopy
2.4. Thermoanalytical Measurements
2.5. Resolvation
2.6. Magnetic Investigations
3. Experimental Section
3.1. General
3.2. Synthesis of Compound 1
3.3. Synthesis of Compound 1-H2O
3.4. Synthesis of Compound 2
3.5. Synthesis of Compound 3
3.6. Synthesis of Compound 4
3.7. Synthesis of Compound 5
3.8. Elemental Analysis
3.9. IR Spectroscopy
3.10. Differential Thermal Analysis and Thermogravimetry
3.11. Single-Crystal Structure Analysis
3.12. X-Ray Powder Diffraction
3.13. Magnetic Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Diaz-Torres, R.; Alvarez, S. Coordinating ability of anions and solvents towards transition metals and lanthanides. Dalton Trans. 2011, 40, 10742–10750. [Google Scholar] [CrossRef] [PubMed]
- Batten Stuart, R.; Champness Neil, R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’Keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal-organic frameworks and coordination polymers (iupac recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar]
- Janiak, C.; Vieth, J.K. Mofs, mils and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Champness, N.R.; Janiak, C. Recent advances in crystal engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Miller, J.S.; Gatteschi, D. Molecule-based magnets. Chem. Soc. Rev. 2011, 40, 3065–3066. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Avendano, C.; Dunbar, K.R. Molecular magnetic materials based on 4d and 5d transition metals. Chem. Soc. Rev. 2011, 40, 3213–3238. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Juan, J.M.; Coronado, E.; Gaita-Arino, A. Magnetic polyoxometalates: From molecular magnetism to molecular spintronics and quantum computing. Chem. Soc. Rev. 2012, 41, 7464–7478. [Google Scholar] [CrossRef] [PubMed]
- Caneschi, A.; Gatteschi, D.; Totti, F. Molecular magnets and surfaces: A promising marriage. A DFT insight. Coord. Chem. Rev. 2015, 289–290, 357–378. [Google Scholar] [CrossRef]
- Liu, K.; Shi, W.; Cheng, P. Toward heterometallic single-molecule magnets: Synthetic strategy, structures and properties of 3d–4f discrete complexes. Coord. Chem. Rev. 2015, 289–290, 74–122. [Google Scholar] [CrossRef]
- Rams, M.; Peresypkina, E.V.; Mironov, V.S.; Wernsdorfer, W.; Vostrikova, K.E. Magnetic relaxation of 1D coordination polymers (x)2[Mn(acacen)Fe(CN)6], X = Ph4P+, Et4N+. Inorg. Chem. 2014, 53, 10291–10300. [Google Scholar] [CrossRef] [PubMed]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Magnetic nanoporous coordination polymers. J. Mater. Chem. 2004, 14, 2713–2723. [Google Scholar] [CrossRef]
- Maspoch, D.; Ruiz-Molina, D.; Veciana, J. Old materials with new tricks: Multifunctional open-framework materials. Chem. Soc. Rev. 2007, 36, 770–818. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Nakabayashi, K.; Arczynski, M.; Pelka, R.; Ohkoshi, S.; Sieklucka, B. Multifunctionality in bimetallic LnIII[WV(CN)8]3− (Ln = Gd, Nd) coordination helices: Optical activity, luminescence, and magnetic coupling. Chem. Eur. J. 2014, 20, 7144–7159. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Sigrist, M.; Sørensen, M.A.; Barra, A.-L.; Weyhermüller, T.; Piligkos, S.; Thuesen, C.A.; Vinum, M.G.; Mutka, H.; Weihe, H.; et al. [ReF6]2−: A robust module for the design of molecule-based magnetic materials. Angew. Chem. 2014, 126, 1375–1378. [Google Scholar] [CrossRef]
- Shurdha, E.; Lapidus, S.H.; Stephens, P.W.; Moore, C.E.; Rheingold, A.L.; Miller, J.S. Extended network thiocyanate- and tetracyanoethanide-based first-row transition metal complexes. Inorg. Chem. 2012, 51, 9655–9665. [Google Scholar] [CrossRef] [PubMed]
- Massoud, S.S.; Quan, L.L.; Gatterer, K.; Albering, J.H.; Fischer, R.C.; Mautner, F.A. Structural characterization of five-coordinate copper(II), nickel(II), and cobalt(II) thiocyanato complexes derived from bis(2-(3,5-dimethyl-1-pyrazolyl)ethyl)amine. Polyhedron 2012, 31, 601–606. [Google Scholar] [CrossRef]
- Małecki, J.G.; Groń, T.; Duda, H. Structural, spectroscopic and magnetic properties of thiocyanate complexes of Mn(II), Ni(II) and Cu(II) with the 1-methylimidazole ligand. Polyhedron 2012, 36, 56–68. [Google Scholar] [CrossRef]
- Wriedt, M.; Jeß, I.; Näther, C. Synthesis, crystal structure, and thermal and magnetic properties of new transition metal-pyrazine coordination polymers. Eur. J. Inorg. Chem. 2009, 1406–1413. [Google Scholar] [CrossRef]
- Létard, J.-F.; Asthana, S.; Shepherd, H.J.; Guionneau, P.; Goeta, A.E.; Suemura, N.; Ishikawa, R.; Kaizaki, S. Photomagnetism of a sym-cis-dithiocyanato iron(II) complex with a tetradentate n,n′-bis(2-pyridylmethyl)1,2-ethanediamine ligand. Chem. Eur. J. 2012, 18, 5924–5934. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Acosta, A.; Chiozzone, R.; Kremer, C.; Armentano, D.; De Munno, G.; Julve, M.; Lloret, F.; Faus, J. New family of thiocyanate-bridged Re(IV)-SCN-M(II) (M = Ni, Co, Fe, and Mn) heterobimetallic compounds: Synthesis, crystal structure, and magnetic properties. Inorg. Chem. 2012, 51, 5737–5747. [Google Scholar] [CrossRef] [PubMed]
- Palion-Gazda, J.; Machura, B.; Lloret, F.; Julve, M. Ferromagnetic coupling through the end-to-end thiocyanate bridge in cobalt(II) and nickel(II) chains. Cryst. Growth Des. 2015, 15, 2380–2388. [Google Scholar] [CrossRef]
- Werner, J.; Rams, M.; Tomkowicz, Z.; Runčevski, T.; Dinnebier, R.E.; Suckert, S.; Näther, C. Thermodynamically metastable thiocyanato coordination polymer that shows slow relaxations of the magnetization. Inorg. Chem. 2015, 54, 2893–2901. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.; Louka, F.; Gallo, A.; Albering, J.; Saber, M.; Burham, N.; Massoud, S. Thiocyanato-copper(II) complexes derived from a tridentate amine ligand and from alanine. Transit. Met. Chem. 2010, 35, 613–619. [Google Scholar] [CrossRef]
- Werner, J.; Runčevski, T.; Dinnebier, R.; Ebbinghaus, S.G.; Suckert, S.; Näther, C. Thiocyanato coordination polymers with isomeric coordination networks—Synthesis, structures, and magnetic properties. Eur. J. Inorg. Chem. 2015, 20, 3236–3245. [Google Scholar] [CrossRef]
- Massoud, S.S.; Dubin, M.; Guilbeau, A.E.; Spell, M.; Vicente, R.; Wilfling, P.; Fischer, R.C.; Mautner, F.A. Azido- and thiocyanato-cobalt(II) complexes based pyrazole ligands. Polyhedron 2014, 78, 135–140. [Google Scholar] [CrossRef]
- Machura, B.; Świtlicka, A.; Zwoliński, P.; Mroziński, J.; Kalińska, B.; Kruszynski, R. Novel bimetallic thiocyanate-bridged Cu(II)–Hg(II) compounds—Synthesis, x-ray studies and magnetic properties. J. Solid State Chem. 2013, 197, 218–227. [Google Scholar] [CrossRef]
- Machura, B.; Świtlicka, A.; Mroziński, J.; Kalińska, B.; Kruszynski, R. Structural diversity and magnetic properties of thiocyanate copper(II) complexes. Polyhedron 2013, 52, 1276–1286. [Google Scholar] [CrossRef]
- Massoud, S.S.; Guilbeau, A.E.; Luong, H.T.; Vicente, R.; Albering, J.H.; Fischer, R.C.; Mautner, F.A. Mononuclear, dinuclear and polymeric 1d thiocyanato- and dicyanamido–copper(II) complexes based on tridentate coligands. Polyhedron 2013, 54, 26–33. [Google Scholar] [CrossRef]
- Werner, J.; Tomkowicz, Z.; Reinert, T.; Näther, C. Synthesis, structure, and properties of coordination polymers with layered transition-metal thiocyanato networks. Eur. J. Inorg. Chem. 2015, 2015, 3066–3075. [Google Scholar] [CrossRef]
- Wöhlert, S.; Runčevski, T.; Dinnebier, R.E.; Ebbinghaus, S.G.; Näther, C. Synthesis, structures, polymorphism, and magnetic properties of transition metal thiocyanato coordination compounds. Cryst. Growth Des. 2014, 14, 1902–1913. [Google Scholar] [CrossRef]
- Mousavi, M.; Bereau, V.; Duhayon, C.; Guionneau, P.; Sutter, J.-P. First magnets based on thiocyanato-bridges. Chem. Commun. 2012, 48, 10028–10030. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Vicente, R.; Massoud, S.S. Synthesis and characterization of five new thiocyanato- and cyanato-metal(II) complexes with 4-azidopyridine as co-ligand. Polyhedron 2015, 85, 20–26. [Google Scholar] [CrossRef]
- Uhrecký, R.; Ondrejkovičová, I.; Lacková, D.; Fáberová, Z.; Mroziński, J.; Kalińska, B.; Padělková, Z.; Koman, M. New thiocyanato iron(II) complex with 3,5-bis(3-pyridyl)-1,2,4-thiadiazole: Synthesis, structure, magnetic and spectral properties. Inorg. Chim. Acta 2014, 414, 33–38. [Google Scholar] [CrossRef]
- Banerjee, S.; Wu, B.; Lassahn, P.-G.; Janiak, C.; Ghosh, A. Synthesis, structure and bonding of cadmium(II) thiocyanate systems featuring nitrogen based ligands of different denticity. Inorg. Chim. Acta 2005, 358, 535–544. [Google Scholar] [CrossRef]
- Werner, J.; Rams, M.; Tomkowicz, Z.; Näther, C. A Co(II) thiocyanato coordination polymer with 4-(3-phenylpropyl)pyridine: The influence of the co-ligand on the magnetic properties. Dalton Trans. 2014, 43, 17333–17342. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, J.; Wriedt, M.; Näther, C. Metamagnetism and single-chain magnetic behavior in a homospin 1d iron(II) coordination polymer. Chem. Eur. J. 2012, 18, 5284–5289. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, S.; Ruschewitz, U.; Näther, C. Metamagnetism and slow relaxation of the magnetization in the 2d coordination polymer: [Co(NCSe)2(1,2-bis(4-pyridyl)ethylene)]n. Cryst. Growth Des. 2012, 12, 2715–2718. [Google Scholar] [CrossRef]
- Näther, C.; Wöhlert, S.; Boeckmann, J.; Wriedt, M.; Jeß, I. A rational route to coordination polymers with condensed networks and cooperative magnetic properties. Z. Anorg. Allg. Chem. 2013, 639, 2696–2714. [Google Scholar] [CrossRef]
- Boeckmann, J.; Näther, C. Metamagnetism and long range ordering in µ-1,3 bridging transition metal thiocyanato coordination polymers. Polyhedron 2012, 31, 587–595. [Google Scholar] [CrossRef]
- Werner, J.; Neumann, T.; Näther, C. Synthesis, structures, and properties of transition metal thiocyanato coordination compounds with 4-(4-chlorobenzyl)pyridine as ligand. Z. Anorg. Allg. Chem. 2014, 640, 2839–2846. [Google Scholar] [CrossRef]
- Wöhlert, S.; Peters, L.; Näther, C. Synthesis, structures and magnetic properties of Fe(II) and Co(II) thiocyanato coordination compounds: On the importance of the diamagnetic counterparts for structure determination. Dalton Trans. 2013, 42, 10746–10758. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, S.; Näther, C. Structures and magnetic properties of nickel thiocyanato coordination compounds with 2-chloropyrazine as a neutral coligand. Eur. J. Inorg. Chem. 2013, 2013, 2528–2537. [Google Scholar] [CrossRef]
- Wöhlert, S.; Wriedt, M.; Fic, T.; Tomkowicz, Z.; Haase, W.; Näther, C. Synthesis, crystal structure and magnetic properties of the coordination polymer [Fe(NCS)2(1,2-bis(4-pyridyl)-ethylene)]n showing a two step metamagnetic transition. Inorg. Chem. 2013, 52, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, S.; Tomkowicz, Z.; Rams, M.; Ebbinghaus, S.G.; Fink, L.; Schmidt, M.U.; Näther, C. Influence of the co-ligand on the magnetic and relaxation properties of layered Co(II) thiocyanato coordination polymers. Inorg. Chem. 2014, 53, 8298–8310. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, S.; Boeckmann, J.; Wriedt, M.; Näther, C. Coexistence of metamagnetism and slow relaxation of magnetization in a cobalt thiocyanate 2d coordination network. Angew. Chem. Int. Ed. 2011, 50, 6920–6923. [Google Scholar] [CrossRef] [PubMed]
- Werner, J.; Tomkowicz, Z.; Rams, M.; Ebbinghaus, S.G.; Neumann, T.; Näther, C. Synthesis, structure and properties of [Co(NCS)2(4-(4-chlorobenzyl)pyridine)2]n, that shows slow magnetic relaxations and a metamagnetic transition. Dalton Trans. 2015, 44, 14149–14158. [Google Scholar] [CrossRef] [PubMed]
- Wöhlert, S.; Fic, T.; Tomkowicz, Z.; Ebbinghaus, S.G.; Rams, M.; Haase, W.; Näther, C. Structural and magnetic studies of a new Co(II) thiocyanato coordination polymer showing slow magnetic relaxations and a metamagnetic transition. Inorg. Chem. 2013, 52, 12947–12957. [Google Scholar] [CrossRef] [PubMed]
- Maroszová, J.; Moncol, J.; Padělková, Z.; Sillanpää, R.; Lis, T.; Koman, M. Self-assembled hydrogen-bonded coordination networks in two copper(II) carboxylates with 4-pyridylmethanol. Cent. Eur. J. Chem. 2011, 9, 453–459. [Google Scholar] [CrossRef]
- Moncol, J.; Mudra, M.; Lonnecke, P.; Koman, M.; Melnik, M. Hydrogen-bonded coordination network in crystal structures of [Cu(3-PM)4Cl2] and [Cu(4-PM)4Cl]Cl, (PM = pyridylmethanol). J. Chem. Crystallogr. 2004, 34, 423–431. [Google Scholar] [CrossRef]
- Moncol, J.; Kalinakova, B.; Svorec, J.; Kleinova, M.; Koman, M.; Hudecova, D.; Melnik, M.; Mazur, M.; Valko, M. Spectral properties and bio-activity of copper(II) clofibriates, part III: Crystal structure of Cu(clofibriate)2(2-pyridylmethanol)2, Cu(clofibriate)2(4-pyridylmethanol)2(H2O) dihydrate, and Cu2(clofibriate)4(n,n-diethylnicotinamide)2. Inorg. Chim. Acta 2004, 357, 3211–3222. [Google Scholar] [CrossRef]
- Stamatatos, T.C.; Foguet-Albiol, D.; Perlepes, S.P.; Raptopoulou, C.P.; Terzis, A.; Patrickios, C.S.; Christou, G.; Tasiopoulos, A.J. 4-(hydroxymethyl)pyridine and pyrimidine in manganese benzoate chemistry: Preparation and characterization of hexanuclear clusters featuring the core. Polyhedron 2006, 25, 1737–1746. [Google Scholar] [CrossRef]
- Goher, M.A.S.; Mautner, F.A.; Gatterer, K.; Abu-Youssef, M.A.M.; Badr, A.M.A.; Sodin, B.; Gspan, C. Four [Cd(L)2(N3)2]n 1d systems with different azide bridging sequences: Synthesis, spectral and structural characterization. J. Mol. Struct. 2008, 876, 199–205. [Google Scholar] [CrossRef]
- Werner, J.; Näther, C. Synthesis, crystal structures and properties of Ni(NCS)2–4-(hydroxymethyl)pyridine coordination compounds. Polyhedron 2015, 98, 96–104. [Google Scholar] [CrossRef]
- Uçar, İ.; Bulut, İ.; Bulut, A.; Karadağ, A. Polymeric and monomeric dipicolinate complexes with 4-hydroxymethyl pyridine: Spectral, structural, thermal and electrochemical characterization. Struct. Chem. 2009, 20, 825–838. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of Shelx. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Bruker, A.X.S. Topas; Version 5.0; Bruker: Karlsruhe, Germany, 2014. [Google Scholar]
- Coelho, A. Whole-profile structure solution from powder diffraction data using simulated annealing. J. Appl. Cryst. 2000, 33, 899–908. [Google Scholar] [CrossRef]










| Compound | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Formula | C26H28CoN6O4S2 | C18H26CoN4O4S2 | C14H18CoN4O4S2 | C14H22CoN4O6S2 |
| MW/g mol−1 | 611.59 | 485.48 | 429.37 | 465.40 |
| crystal system | orthorhombic | monoclinic | orthorhombic | triclinic |
| space group | P212121 | C2/c | Pbca | |
| a/Å | 11.5440(3) | 9.0056(4) | 12.3007(5) | 7.3858(7) |
| b/Å | 14.3213(3) | 16.1092(7) | 7.7432(5) | 7.6952(6) |
| c/Å | 17.6437(4) | 16.3412(8) | 19.3263(14) | 9.4172(9) |
| β/° | 90 | 95.446(4) | 90 | 70.828(10) |
| V/Å3 | 2916.95(12) | 2359.97(19) | 1840.77(16) | 491.53(8) |
| T/K | 200(2) | 200(2) | 200(2) | 200(2) |
| Z | 4 | 4 | 4 | 1 |
| Dcalc/mg·cm−3 | 1.393 | 1.366 | 1.549 | 1.572 |
| µ/mm−1 | 0.773 | 0.933 | 1.1185 | 1.123 |
| θmax/deg | 1.83 to 27.96 | 2.53 to 27.51 | 2.11 to 27.94 | 2.35 to 27.91 |
| measured refl. | 31835 | 11473 | 13046 | 5125 |
| unique refl. | 6972 | 2700 | 2190 | 2277 |
| Refl. (F0 > 4σ(F0)) | 6397 | 2299 | 1808 | 1952 |
| parameter | 356 | 151 | 116 | 126 |
| Rint | 0.0525 | 0.0402 | 0.0441 | 0.0335 |
| R1 (F0 > 4σ(F0)) | 0.0343 | 0.0514 | 0.0473 | 0.0406 |
| wR2 (all data) | 0.0708 | 0.1217 | 0.1134 | 0.1115 |
| GOF | 1.106 | 1.074 | 1.132 | 1.043 |
| Δρmax/min/e Å−3 | 0.276/−0.322 | 0.710/−0.803 | 0.475/−0.527 | 0.541/−0.555 |
| Compound | 1-H2O | 5 |
|---|---|---|
| formula | C26H31.4CoN6O5.7S2 | C14H14CoN4O2S2 |
| MW/g mol−1 | 641.83 | 393.35 |
| Crystal system | cubic | monoclinic |
| Space group | :2 | P21/c |
| a/Å | 16.7494(6) | 10.7088(6) |
| b/Å | 16.7494(6) | 20.2164(11) |
| c/Å | 16.7494(6) | 7.9016(4) |
| α/° | 90 | 90 |
| β/° | 90 | 107.181(3) |
| γ/° | 90 | 90 |
| V/Å3 | 4698.9(5) | 1634.32(15) |
| T/K | 293 (2) | 293 (2) |
| Z | 6 | 4 |
| Dcalc/mg·cm−3 | 1.355 | 1.599 |
| µ/mm−1 | 0.7468 | 1.35197 |
| λ/Å | 0.7093 | 0.7093 |
| θmax/deg | 2.00 to 49.80 | 2 to 49.80 |
| Rwp/% a | 5.54 | 4.84 |
| Rp/% a | 4.33 | 3.66 |
| Rexp/% a | 1.76 | 1.67 |
| RBragg/% a | 4.01 | 2.89 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suckert, S.; Germann, L.S.; Dinnebier, R.E.; Werner, J.; Näther, C. Synthesis, Structures and Properties of Cobalt Thiocyanate Coordination Compounds with 4-(hydroxymethyl)pyridine as Co-ligand. Crystals 2016, 6, 38. https://doi.org/10.3390/cryst6040038
Suckert S, Germann LS, Dinnebier RE, Werner J, Näther C. Synthesis, Structures and Properties of Cobalt Thiocyanate Coordination Compounds with 4-(hydroxymethyl)pyridine as Co-ligand. Crystals. 2016; 6(4):38. https://doi.org/10.3390/cryst6040038
Chicago/Turabian StyleSuckert, Stefan, Luzia S. Germann, Robert E. Dinnebier, Julia Werner, and Christian Näther. 2016. "Synthesis, Structures and Properties of Cobalt Thiocyanate Coordination Compounds with 4-(hydroxymethyl)pyridine as Co-ligand" Crystals 6, no. 4: 38. https://doi.org/10.3390/cryst6040038
APA StyleSuckert, S., Germann, L. S., Dinnebier, R. E., Werner, J., & Näther, C. (2016). Synthesis, Structures and Properties of Cobalt Thiocyanate Coordination Compounds with 4-(hydroxymethyl)pyridine as Co-ligand. Crystals, 6(4), 38. https://doi.org/10.3390/cryst6040038