The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding
Abstract
:1. Introduction
2. Results and Discussion
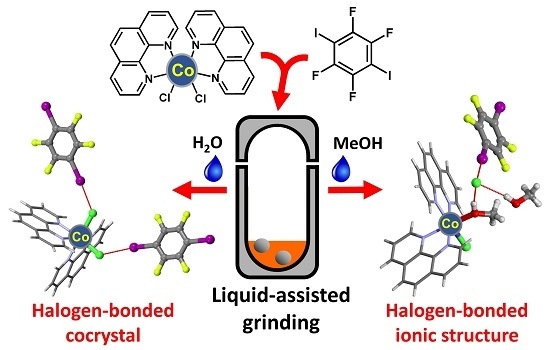
2.1. Syntheses
2.2. Structural Analysis
3. Materials and Methods
3.1. Synthesis of Complexes
3.2. Mechanochemical Synthesis of 1
3.3. Mechanochemical Synthesis of 2
3.4. Crystallization of 1 and 2
3.5. Thermal Analysis
3.6. Single Crystal X-ray Diffraction Experiments
3.7. Powder X-ray Diffraction Experiments
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Desiraju, G.R. Crystal engineering: A holistic view. Angew. Chem. Int. Ed. 2007, 46, 8342–8356. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Salmon, D.J. Building co-crystals with molecular sense and supramolecular sensibility. CrystEngComm 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Friščić, T. Supramolecular concepts and new techniques in mechanochemistry: Cocrystals, cages, rotaxanes, open metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 3493–3510. [Google Scholar] [CrossRef] [PubMed]
- Hassel, O. Structural aspects of interatomic charge-transfer bonding. Science 1970, 170, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimägi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Priimägi, A.; Cavallo, G.; Metrangolo, P.; Resnati, G. The Halogen Bond in the Design of Functional Supramolecular Materials: Recent Advances. Acc. Chem. Res. 2013, 46, 2686–2695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fourmigué, M. Halogen bonding: Recent advances. Curr. Opin. Solid State Mater. Sci. 2009, 13, 36–45. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Isostructural Materials Achieved by Using Structurally Equivalent Donors and Acceptors in Halogen-Bonded Cocrystals. Chem. Eur. J. 2008, 14, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnati, G. Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Stilinović, V.; Horvat, G.; Hrenar, T.; Nemec, V.; Cinčić, D. Halogen and Hydrogen Bonding between (N-Halogeno)-succinimides and Pyridine Derivatives in Solution, the Solid State and In Silico. Chem. Eur. J. 2017, 22, 5244–5257. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Li, B.; Zhang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Gamekkanda, J.C.; Sinha, A.S.; Desper, J.; Ðaković, M.; Aakeröy, C.B. The Role of Halogen Bonding in Controlling Assembly and Organization of Cu (II)-Acac Based Coordination Complexes. Crystals 2017, 7, 226. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Schultheiss, N.; Desper, J.; Moore, C. Attempted assembly of discrete coordination complexes into 1-D chains using halogen bonding or halogen⋯halogen interactions. CrystEngComm 2007, 9, 421–426. [Google Scholar] [CrossRef]
- Johnson, M.T.; Džolić, Z.; Cetina, M.; Wendt, O.F.; Ohrstrom, L.; Rissanen, K. Neutral Organometallic Halogen Bond Acceptors: Halogen Bonding in Complexes of PCPPdX (X = Cl, Br, I) with Iodine (I2), 1,4-Diiodotetrafluorobenzene (F4DIBz), and 1,4-Diiodooctafluorobutane (F8DIBu). Cryst. Growth Des. 2012, 12, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Christopherson, J.-C.; Potts, K.P.; Bushuyev, O.S.; Topić, F.; Huskić, I.; Rissanen, K.; Barrett, C.J.; Friščić, T. Assembly and dichroism of a four-component halogen-bonded metal-organic cocrystal salt solvate involving dicyanoaurate(I) acceptors. Faraday Discuss. 2017, 203, 441–457. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tuikka, M.J.; Hirva, P.; Kukushkin, V.Y.; Novikov, A.S.; Haukka, M. Fine-tuning halogen bonding properties of diiodine through halogen–halogen charge transfer—Extended [Ru(2,2′-bipyridine)(CO)2X2]·I2 systems (X = Cl, Br, I). CrystEngComm 2016, 18, 1987–1995. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T. Synthesis of an extended halogen-bonded metal–organic structure in a one-pot mechanochemical reaction that combines covalent bonding, coordination chemistry and supramolecular synthesis. CrystEngComm 2014, 16, 10169–10172. [Google Scholar] [CrossRef]
- Lapadula, G.; Judaš, N.; Friščić, T.; Jones, W. A Three-Component Modular Strategy to Extend and Link Coordination Complexes by Using Halogen Bonds to O, S and π Acceptors. Chem. Eur. J. 2010, 16, 7400–7403. [Google Scholar] [CrossRef] [PubMed]
- Nemec, V.; Fotović, L.; Friščić, T.; Cinčić, D. A Large Family of Halogen-Bonded Cocrystals Involving Metal–Organic Building Blocks with Open Coordination Sites. Cryst. Growth Des. 2017. [Google Scholar] [CrossRef]
- Chen, X.; Han, S.; Wang, R.; Li, Y. Four supramolecular isomers of dichloridobis(1,10-phenanthroline)cobalt(II): Synthesis, structure characterization and isomerization. Acta Crystallogr. Sect. C 2016, 72, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-L.; Liu, D.-X.; Liu, T.-F. A polymorph of cis-dichloridobis(1,10-phenanthroline-κ2N,N′)cobalt(II). Acta Crystallogr. Sect. E 2007, 63, m1880. [Google Scholar] [CrossRef]
- Moulton, B.; Zaworotko, M.J. From Molecules to Crystal Engineering: Supramolecular Isomerism and Polymorphism in Network Solids. Chem. Rev. 2001, 101, 1629–1658. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Tong, M.; Chen, X. Metal-organic molecular architectures with 2,2′-bipyridyl-like and carboxylate ligands. Coord. Chem. Rev. 2005, 249, 545–565. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. B 2016, B72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Braga, D.; Curzi, M.; Johansson, A.; Polito, M.; Rubini, K.; Grepioni, F. Simple and Quantitative Mechanochemical Preparation of a Porous Crystalline Material Based on a 1D Coordination Network for Uptake of Small Molecules. Angew. Chem. Int. Ed. 2006, 45, 148–152. [Google Scholar] [CrossRef]
- Friščić, T.; Childs, S.L.; Rizvi, S.A.A.; Jones, W. The role of solvent in mechanochemical and sonochemical cocrystal formation: A solubility-based approach for predicting cocrystallisation outcome. CrystEngComm 2009, 11, 418–426. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-W. Mechanochemical organic synthesis. Chem. Soc. Rev. 2013, 42, 7668–7700. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T. New opportunities for materials synthesis using mechanochemistry. J. Mater. Chem. 2010, 20, 7599–7605. [Google Scholar] [CrossRef]
- Braga, D.; Maini, L.; Grepioni, F. Mechanochemical preparation of co-crystals. Chem. Soc. Rev. 2013, 42, 7638–7648. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, E.V. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Kaitner, B. Schiff base derived from 2-hydroxy-1-naphthaldehyde and liquid-assisted mechanochemical synthesis of its isostructural Cu(II) and Co(II) complexes. CrystEngComm 2011, 13, 4351–4357. [Google Scholar] [CrossRef]
- Šepelák, V.; Düvel, A.; Wilkening, M.; Becker, K.-D.; Heitjans, P. Mechanochemical reactions and syntheses of oxides. Chem. Soc. Rev. 2013, 42, 7507–7520. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Brekalo, I.; Kaitner, B. Effect of atmosphere on solid-state amine-aldehyde condensations: Gas-phase catalysts for solid-state transformations. Chem. Commun. 2012, 48, 11683–11685. [Google Scholar] [CrossRef] [PubMed]
- Cinčić, D.; Juribašić, M.; Babić, D.; Molčanov, K.; Šket, P.; Plavec, J.; Ćurić, M. New insight into solid-state molecular dynamics: Mechanochemical synthesis of azobenzene/triphenylphosphine palladacycles. Chem. Commun. 2011, 47, 11543–11545. [Google Scholar] [CrossRef] [PubMed]
- Baláž, P.; Achimovičová, M.; Baláž, M.; Billik, P.; Cherkezova-Zheleva, Z.; Criado, J.M.; Delogu, F.; Dutková, E.; Gaffet, E.; José Gotor, F.; et al. Hallmarks of mechanochemistry: From nanoparticles to technology. Chem. Soc. Rev. 2013, 42, 7571–7637. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Stilinović, V.; Cinčić, D.; Zbačnik, M.; Kaitner, B. Controlling solvate formation of a Schiff base by combining mechanochemistry with solution synthesis. Croat. Chem. Acta 2012, 85, 485–493. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F. Making crystals from crystals: A green route to crystal engineering and polymorphism. Chem. Commun. 2005, 3635–3645. [Google Scholar] [CrossRef] [PubMed]
- Springuel, G.; Robeyns, K.; Norberg, B.; Wouters, J.; Leyssens, T. Cocrystal Formation between Chiral Compounds: How Cocrystals Differ from Salts. Cryst. Growth Des. 2014, 14, 3996–4004. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. A Stepwise Mechanism for the Mechanochemical Synthesis of Halogen-Bonded Cocrystal Architectures. J. Am. Chem. Soc. 2008, 130, 7524–7525. [Google Scholar] [CrossRef]
- Cinčić, D.; Friščić, T.; Jones, W. Structural Equivalence of Br and I Halogen Bonds: A Route to Isostructural Materials with Controllable Properties. Chem. Mater. 2008, 20, 6623–6626. [Google Scholar] [CrossRef]
- Mavračić, J.; Cinčić, D.; Kaitner, B. Halogen bonding of N-bromosuccinimide by grinding. CrystEngComm 2016, 18, 3343–3346. [Google Scholar] [CrossRef]
- Nemec, V.; Cinčić, D. Uncommon halogen bond motifs in cocrystals of aromatic amines and 1,4-diiodotetrafluorobenzene. CrystEngComm 2016, 18, 7425–7429. [Google Scholar] [CrossRef]
- Brammer, L.; Bruton, E.A.; Sherwood, P. Understanding the Behavior of Halogens as Hydrogen Bond Acceptors. Cryst. Growth Des. 2001, 1, 277–290. [Google Scholar] [CrossRef]
- Stilinović, V.; Užarević, K.; Cvrtila, I.; Kaitner, B. Bis(morpholine) hydrogen bond pincer—A novel series of heteroleptic Cu(II) coordination compounds as receptors for electron rich guests. CrystEngComm 2012, 14, 7493–7501. [Google Scholar] [CrossRef]
- Pfrunder, M.C.; Micallef, A.S.; Rintoul, L.; Arnold, D.P.; McMurtrie, J. Interplay between the Supramolecular Motifs of Polypyridyl Metal Complexes and Halogen Bond Networks in Cocrystals. Cryst. Growth Des. 2016, 16, 681–695. [Google Scholar] [CrossRef]
- Cavallo, G.; Biella, S.; Lu, J.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Halide anion-templated assembly of di- and triiodoperfluorobenzenes into 2D and 3D supramolecular networks. J. Fluor. Chem. 2010, 131, 1165–1172. [Google Scholar] [CrossRef]
- Raatikainen, K.; Rissanen, K. Modulation of N···I and +N−H···Cl−···I Halogen Bonding: Folding, Inclusion, and Self-Assembly of Tri- and Tetraamino Piperazine Cyclophanes. Cryst. Growth Des. 2016, 10, 3638–3646. [Google Scholar] [CrossRef]
- STARe Software v.14.00; MettlerToledo GmbH: Giessen, Germany, 2015.
- Oxford Diffraction. Xcalibur CCD System, CrysAlis CCD and CrysAlis RED, version 1.171; Oxford Diffraction Ltd.: Abingdon, UK, 2008. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; Streek, J.V.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Philips X’Pert Data Collector 1.3e; Philips Analytical B. V.: Almelo, The Netherlands, 2001.
- Philips X’Pert Graphic & Identify 1.3e Philips; Analytical B. V.: Almelo, The Netherlands, 2001.
- Philips X’Pert Plus 1.0; Philips Analytical B. V.: Almelo, The Netherlands, 1999.





| Compound 1 | Compound 2 | |
|---|---|---|
| Molecular Formula | (CoCl2C24H16N4)(C6F4I2) | [(CoClC25H20N4O)]Cl(CH3OH)(C6F4I2) |
| Mr | 892.10 | 956.18 |
| Crystal system | triclinic | triclinic |
| Space group | P | P |
| Crystal data: | ||
| a/Å | 9.9129(5) | 9.1111(3) |
| b/Å | 12.8302(5) | 11.1583(5) |
| c/Å | 14.0185(6) | 17.8104(8) |
| α/° | 116.357(4) | 77.661(4) |
| β/° | 103.424(4) | 76.942(4) |
| γ/° | 96.119(4) | 81.311(3) |
| V/Å3 | 1508.31(13) | 1713.04(13) |
| Z | 2 | 2 |
| Dcalc/g cm−3 | 1.964 | 1.854 |
| λ(MoKα)/Å | 0.71073 | 0.71073 |
| T/K | 295 | 295 |
| Crystal size/mm3 | 0.46 × 0.25 × 0.11 | 0.60 × 0.55 × 0.31 |
| µ/mm−1 | 2.846 | 2.517 |
| F(000) | 854 | 926 |
| Refl. collected/unique | 6632/4212 | 5872/4242 |
| Parameters | 388 | 430 |
| Δρmax, Δρmin/e Å−3 | 0.547; −0.632 | 0.586; −0.449 |
| R[F2 > 4σ(F2)] | 0.0294 | 0.0297 |
| wR(F2) | 0.0772 | 0.0655 |
| Goodness-of-fit, S | 0.879 | 0.940 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisac, K.; Cinčić, D. The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding. Crystals 2017, 7, 363. https://doi.org/10.3390/cryst7120363
Lisac K, Cinčić D. The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding. Crystals. 2017; 7(12):363. https://doi.org/10.3390/cryst7120363
Chicago/Turabian StyleLisac, Katarina, and Dominik Cinčić. 2017. "The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding" Crystals 7, no. 12: 363. https://doi.org/10.3390/cryst7120363
APA StyleLisac, K., & Cinčić, D. (2017). The Influence of Liquid on the Outcome of Halogen-Bonded Metal–Organic Materials Synthesis by Liquid Assisted Grinding. Crystals, 7(12), 363. https://doi.org/10.3390/cryst7120363