Synthesis, Crystal Structure, Magnetic Property and Photo-Induced Coloration of a One-Dimensional Chain Complex
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of [Co(HDDCPBA)0.5(Phen)(H2O)2]·2H2O (1)
2.3. X-Ray Crystallography
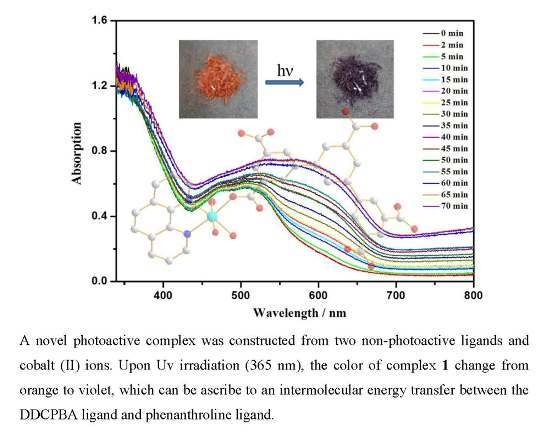
3. Results and Discussion
3.1. Descriptions of the Crystal Structures
3.2. The Diffuse-Reflectance Spectra and ESR Spectra
3.3. X-Ray Powder Diffraction Analysis
3.4. IR Spectra
3.5. Thermogravimetric Analyses
3.6. Magnetic Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sangeetha, N.M.; Maitra, U. Supramolecular gels: Functions and uses. Chem. Soc. Rev. 2005, 34, 821. [Google Scholar] [CrossRef] [PubMed]
- Li, C.P.; Du, M. Role of solvents in coordination supramolecular systems. Chem. Commun. 2011, 47, 5958. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, Q.X.; Zeng, M.H. Iodine Release and Recovery, Influence of Polyiodide Anions on Electrical Conductivity and Nonlinear Optical Activity in an Interdigitated and Interpenetrated Bipillared-Bilayer Metal−Organic Framework. J. Am. Chem. Soc. 2012, 134, 4857–4863. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Xiang, S.; Qian, G. Metal-Organic Frameworks with Functional Pores for Recognition of Small Molecules. Acc. Chem. Res. 2010, 43, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- O’Keeffe, M.; Yaghi, O.M. Deconstructing the Crystal Structures of Metal Organic Frameworks and Related Materials into Their Underlying Nets. Chem. Rev. 2012, 112, 675–702. [Google Scholar] [CrossRef] [PubMed]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; el Gabaly, F.; Yoon, H.P.; et al. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.P.; Liu, J.; Zhan, S.Z.; Yang, J.R.; Li, D.; Ng, K.M.; Sun, R.W.Y.; Che, C.M. A High-Symmetry Coordination Cage from 38- or 62-Component Self-Assembly. J. Am. Chem. Soc. 2012, 134, 8042–8045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Liu, F.L.; Zhang, L.L.; Sun, D.; Wang, R.M.; Ju, Z.F.; Yuan, D.Q.; Sun, D.F. Achieving a Rare Breathing Behavior in a Polycatenated 2 D to 3 D Net through a Pillar-Ligand Extension Strategy. Chem. Eur. J. 2014, 20, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Li, F.F.; Neti, V.; Metta-Magana, A.J.; Echegoyen, L. Design, Synthesis, and X-Ray Crystal Structure of a Fullerene-Linked Metal–Organic Framework. Angew. Chem. Int. Ed. 2014, 53, 160–163. [Google Scholar] [CrossRef] [PubMed]
- Tai, X.; Wang, X.; You, H. Synthesis, Crystal Structure and Antitumor Activity of a New Zn(II) Complex Based on NAcetyl-L-phenylalanine and 1,10-Phenanthroline. Chin. J. Struct. Chem. 2016, 35, 586–590. [Google Scholar]
- Zhang, L.; Kang, Z.; Xin, X.; Sun, D. Metal–organic frameworks based luminescent materials for nitroaromatics sensing. CrystEngComm 2016, 18, 193–206. [Google Scholar] [CrossRef]
- Zeng, Y.; Fu, Z.; Chen, H.; Liu, C.; Liao, S.; Dai, J. Photo- and thermally induced coloration of a crystalline MOF accompanying electron transfer and long-lived charge separation in a stable host–guest system. Chem. Commun. 2012, 48, 8114–8116. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Liao, S.; Dai, J.; Fu, Z. Fluorescent and photochromic bifunctional molecular switch based on a stable crystalline metal-viologen complex. Chem. Commun. 2012, 48, 11641–11643. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, G.; Li, M.; Wang, Y.; Song, Y.; Han, C.; Dai, J.; Fu, Z. Photo-and thermo-activated electron transfer system based on a luminescent europium organic framework with spectral response from UV to visible range. Chem. Commun. 2014, 50, 13544–13546. [Google Scholar] [CrossRef] [PubMed]
- Easun, T.L.; Jia, J.; Reade, T.J.; Sun, X.; Davies, S.E.; Blake, A.J.; George, M.W.; Champness, N.R. Modification of coordination networks through a photoinduced charge transfer process. Chem. Sci. 2014, 5, 539–544. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Chen, Y.; Zhang, J.; Liao, S.J. Correlation between the photoactive character and the structures of two novel metal organic frameworks. J. Mater. Chem. 2011, 21, 7895–7897. [Google Scholar] [CrossRef]
- Bruker AXS Inc. SMART, SAINT and SADABS; Bruker AXS Inc.: Madison, WI, USA, 1998. [Google Scholar]
- Sheldrick, G.M. SHELXS-97, Program for X-ray Crystal Structure Determination; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, Program for X-ray Crystal Structure Refinement; University of Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Spek, A.L. PLATON, A Multipurpose Crystallographic Tool; Utrecht University: Utrecht, Netherlands, 2002. [Google Scholar]
- Jhang, P.C.; Chuang, N.T.; Wang, S.L. Layered Zinc Phosphates with Photoluminescence and Photochromism: Chemistry in Deep Eutectic Solvents. Angew. Chem. Int. Ed. 2010, 49, 4200–4204. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, G.C.; Guo, J.S.; Guo, S.P.; Jiang, X.M.; Yang, C.; Wang, M.S.; Zhang, Z.J. Photochromic inorganic–organic hybrid: A new approach for switchable photoluminescence in the solid state and partial photochromic phenomenon. Dalton Trans. 2010, 39, 8688–8692. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds; John Wiley & Sons: New York, NY, USA, 1986. [Google Scholar]
- Zhang, L.; Guo, J.; Meng, Q.; Wang, R.; Sun, D. Syntheses, structures and characteristics of four metal–organic coordination polymers based on 5-hydroxyisophthalic acid and N-containing auxiliary ligands. CrystEngComm 2013, 15, 9578–9587. [Google Scholar] [CrossRef]
- Rodríguez, A.; Kivekäs, R.; Colacio, E. Unique self-assembled 2D metal-tetrazolate networks: Crystal structure and magnetic properties of [M(pmtz)2](M = Co(II) and Fe(II); Hpmtz = 5-(pyrimidyl)tetrazole). Chem. Commun. 2005, 41, 5228–5230. [Google Scholar] [CrossRef] [PubMed]








| Empirical Formula | C23.5H20.5CoN2O9 |
|---|---|
| Formula weight | 533.85 |
| Temperature/K | 294.49(10) |
| Crystal system | monoclinic |
| Space group | P2/n |
| a/Å | 7.7636(2) |
| b/Å | 13.8680(4) |
| c/Å | 21.1105(6) |
| α/° | 90.00 |
| β/° | 94.644(3) |
| γ/° | 90.00 |
| Volume/Å3 | 2265.42(11) |
| Z | 4 |
| ρcalcmg/mm3 | 1.565 |
| m/mm−1 | 6.464 |
| F(000) | 1098.0 |
| Index ranges | −9 ≤ h ≤ 9, −16 ≤ k ≤ 14, −25 ≤ l ≤ 18 |
| Reflections collected | 8335 |
| Independent reflections | 4036[R(int) = 0.0315] |
| Data/restraints/parameters | 4036/0/331 |
| Goodness-of-fit on F2 | 1.053 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0503, wR2 = 0.1398 |
| Final R indexes [all data] | R1 = 0.0667, wR2 = 0.1505 |
| Largest diff. peak/hole/e Å−3 | 1.31/−0.40 |
| Co1-O1 | 2.066(2) | Co1-O2 | 2.138(2) | Co1-O3 | 2.103(3) |
|---|---|---|---|---|---|
| Co1-O6 | 2.127(3) | Co1-N14 | 2.118(3) | Co1-N15 | 2.141(3) |
| O1-Co1-O2 | 88.93(9) | O1-Co1-O3 | 89.25(11) | O1-Co1-O6 | 94.77(10) |
| O1-Co1-N14 | 92.63(10) | O1-Co1-N15 | 170.56(11) | O2-Co1-N15 | 91.35(10) |
| O3-Co1-O2 | 174.22(11) | O3-Co1-O6 | 86.05(12) | O3-Co1-N14 | 94.31(12) |
| O3-Co1-N15 | 91.35(11) | O6-Co1-O2 | 88.63(11) | O6-Co1-N15 | 94.67(11) |
| N14-Co1-O2 | 91.26(10) | N14-Co1-O6 | 172.60(11) | N14-Co1-N15 | 77.93(12) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Wang, S.; Zhang, J.; Xun, R.; Chen, M.; Lu, J. Synthesis, Crystal Structure, Magnetic Property and Photo-Induced Coloration of a One-Dimensional Chain Complex. Crystals 2017, 7, 77. https://doi.org/10.3390/cryst7030077
Meng Q, Wang S, Zhang J, Xun R, Chen M, Lu J. Synthesis, Crystal Structure, Magnetic Property and Photo-Induced Coloration of a One-Dimensional Chain Complex. Crystals. 2017; 7(3):77. https://doi.org/10.3390/cryst7030077
Chicago/Turabian StyleMeng, Qingguo, Suqing Wang, Junhao Zhang, Rui Xun, Mingchao Chen, and Jitao Lu. 2017. "Synthesis, Crystal Structure, Magnetic Property and Photo-Induced Coloration of a One-Dimensional Chain Complex" Crystals 7, no. 3: 77. https://doi.org/10.3390/cryst7030077
APA StyleMeng, Q., Wang, S., Zhang, J., Xun, R., Chen, M., & Lu, J. (2017). Synthesis, Crystal Structure, Magnetic Property and Photo-Induced Coloration of a One-Dimensional Chain Complex. Crystals, 7(3), 77. https://doi.org/10.3390/cryst7030077