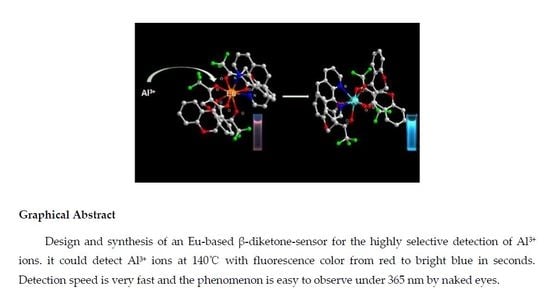
Design and Synthesis of an Eu-Based β-Diketone-Sensor for the Detection of Al3+ Ions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of 2,2,2-Trifluoro-1-(4-hydroxy-2H-chromen-3-yl)ethanone (4-TFC)
2.2. Synthesis of Eu(4-TFC)3(phen) (1)
2.3. X-ray Crystallography
2.4. UV-Vis Spectra Analysis
2.5. Excitation Spectra and Emission Spectra
2.6. Luminescent Properties
2.7. Computational Studies
3. Materials and Instrumentation
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhao, J.; Wang, Y.N.; Dong, W.W.; Wu, Y.P.; Li, D.S.; Zhang, Q.C. A Robust Luminescent Tb(III)-MOF with Lewis Basic Pyridyl Sites for the Highly Sensitive Detection of Metal Ions and Small Molecules. Inorg. Chem. 2016, 55, 3265–3271. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Tian, D.; Gao, Q.; Sun, H.W.; Xu, J.; Bu, X.H. A chiral lanthanide metal–organic framework for selective sensing of Fe(iii) ions. Dalton Trans. 2016, 45, 1040–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Qin, W.; Tang, X.; Dou, W.; Liu, W.; Teng, Q.; Yao, X. A selective, cell-permeable fluorescent probe for Al3+ in living cells. Org. Biomol. Chem. 2010, 8, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Ashton, T.D.; Jolliffe, K.A.; Pfeffer, F.M. Luminescent probes for the bioimaging of small anionic species in vitro and in vivo. Chem. Soc. Rev. 2015, 44, 4547–4595. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Ki, H.L. Optical sensor: A promising strategy for environmental and biomedical monitoring of ionic species. RSC Adv. 2015, 5, 72150–72287. [Google Scholar] [CrossRef]
- Marek, P.; Oksana, P.; Jerzy, K.; Alina, M.; Grzegorz, D.; Teresa, B.; Anna, M.K.; Rik, V.D. Highly photoluminescent europium tetraphenylimidodiphosphinate ternary complexes with heteroaromatic co-ligands. Solution and solid state studies. J. Lumin. 2016, 170, 411–419. [Google Scholar] [CrossRef]
- Stephen, C.B. Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 2016, 52, 222–229. [Google Scholar] [CrossRef]
- Yahya, A.K.; Xinsen, S.; Timothy, J.P.; Mark, R.J.E.; Carl, R. Tetraphenolate niobium and tantalum complexes for the ring opening polymerization of epsilon-caprolactone. Dalton Trans. 2015, 44, 12349–12356. [Google Scholar] [CrossRef]
- Paula, G.; Vladimir, M.A.; Victor, A.T.; Dagmara, K.; Janina, L. The ligand-to-metal energy transfer and the role of Lewis base ligands and silver plasmons in emission of new type of lanthanide phosphors. J. Lumin. 2016, 170, 340–347. [Google Scholar] [CrossRef]
- Wen, J.Z.; Hai, Y.W. Preparation and luminescent properties of lanthanide (Eu3+ and Tb3+) complexes grafted to 3-aminopropyltriethoxysilane by covalent bonds. Opt. Mater. 2015, 50, 208–214. [Google Scholar] [CrossRef]
- Martín, R.P.; Martín, I.R.; Lahoz, P.; Hernández, N.S.; Pereira, S.P.S.; Hernández, I.; Lavín, V.; Ramos, S.M. An erbium(III)-based NIR emitter with a highly conjugated β-diketonate for blue-region sensitization. J. Alloy Compd. 2015, 619, 553–559. [Google Scholar] [CrossRef]
- Wei, G.T.; Yi, N.X.; Jin, T.T.; Pei, Z.G.; Jin, H.D.; Xin, W.; Zhi, B.Z. Colorimetric and fluorometric dual-mode detection of aniline pollutants based on spiropyran derivatives. RSC Adv. 2016, 6, 83312–83320. [Google Scholar] [CrossRef]
- Petra, G.; Romana, C.K.; Maja, V.; Boris, S. Crystal Structures and Emission Properties of the BF2 Complex 1-Phenyl-3-(3,5-dimethoxyphenyl)-propane-1,3-dione: Multiple Chromisms, Aggregation- or Crystallization-Induced Emission, and the Self-Assembly Effect. J. Am. Chem. Soc. 2014, 136, 7383–7394. [Google Scholar] [CrossRef]
- Petri, A.T.; Jouko, J.V.; Sirpa, P. Advanced material and approach for metal ions removal from aqueous solutions. Sci. Rep. 2015, 5, 8992. [Google Scholar] [CrossRef]
- Wei, H.B.; Zhao, Z.F.; Wei, C.; Yu, G.; Liu, Z.W.; Zhang, B.; Bian, J.; Bian, Z.Q.; Huang, C.H. Antiphotobleaching: A Type of Structurally Rigid Chromophore Ready for Constructing Highly Luminescent and Highly Photostable Europium Complexes. Adv. Funct. Mater. 2016, 26, 2085–2096. [Google Scholar] [CrossRef]
- Dennison, G.H.; Johnston, M.R. Mechanistic Insights into the Luminescent Sensing of Organophosphorus Chemical Warfare Agents and Simulants Using Trivalent Lanthanide Complexes. Chem. Eur. J. 2015, 21, 6328–6338. [Google Scholar] [CrossRef] [PubMed]
- Azzazy, H.M.; Mansour, M.M.; Kazmierczak, S.C. From diagnostics to therapy: Prospects of quantum dots. Clin. Biochem. 2007, 40, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Song, R.; Yu, J.; Liu, M.; Wang, Z.; Wu, C.; Yang, Y.; Wang, Z.; Chen, B.; Qian, G. Dual-Emitting MOF ⊃ Dye Composite for Ratiometric Temperature Sensing. Adv. Mater. 2015, 27, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Chatterjee, N.; Bharadwaj, P.K. Selectively sensing first-row transition metal ions through fluorescence enhancement. RSC Adv. 2014, 4, 26585–26620. [Google Scholar] [CrossRef]
- Li, D.; Zhou, M.; Xie, L.; Yu, X.; Yu, Y.; Ai, H.; Tang, S. Synergism of pentaerythritol-zinc with β-diketone and calcium stearate in poly(vinyl chloride) thermal stability. Polym. J. 2013, 45, 775–782. [Google Scholar] [CrossRef]
- Gao, B.; Chen, L.; Chen, T. Effect of electron-donating substituent groups on aromatic ring on photoluminescence properties of complexes of benzoic acid-functionalized polysulfone with Eu(III) ions. Phys. Chem. Chem. Phys. 2015, 17, 25322–25332. [Google Scholar] [CrossRef] [PubMed]
- Reis, S.G.; Briganti, M.; Martins, D.O.; Akpinar, H.; Calancea, S.; Guedes, G.P.; Soriano, S.; Andruh, M.; Cassaro, R.A.A.; Lahti, P.M.; et al. First coordination compounds based on a bis-(imino nitroxide) biradical and 4f metal ions: synthesis, crystal structures and magnetic properties. Dalton Trans. 2016, 45, 2936–2944. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chowdhury, B.; Ghosh, P. A Highly Sensitive ESIPT-Based Ratiometric Fluorescence Sensor for Selective Detection of Al3+. Inorg. Chem. 2016, 55, 9212–9220. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.M.F.; Chen, C.T.; Chen, Y.C. Photoluminescence Determination of Aluminum Using Glutathione-Capped Gold Nanoclusters. Anal. Lett. 2016, 14, 2246–2258. [Google Scholar] [CrossRef]
- Chen, W.W.; Jia, Y.X.; Feng, Y.; Zheng, W.S.; Wang, W.; Jiang, X.Y. Colorimetric detection of Al (III) in vermicelli samples based on ionic liquid group coated gold nanoparticles. RSC Adv. 2015, 5, 62260–62264. [Google Scholar] [CrossRef]
- Miura, Y.; Hiraiwa, M.N.; Ito, T.; Itonaga, T.; Watanabe, Y.; Okabe, S. Bacterial community structures in MBRs treating municipal wastewater: Relationship between community stability and reactor performance. Water Res. 2007, 41, 627–637. [Google Scholar] [CrossRef] [PubMed]
- APEX2, version 2009.9; Bruker AXS Inc.: Tokyo, Japan, 2009.
- SAINT, version 7.68A; Bruker AXS Inc.: Tokyo, Japan, 2009.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.-J.; Trucks, G.-W.; Schlegel, H.-B.; Scuseria, G.-E.; Robb, M.-A.; Cheeseman, J.-R.; Montgomery, J.-A., Jr.; Vreven, T.; Kudin, K.-N.; Burant, J.-C.; et al. Gaussian 03Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Zhang, J.; Li, B.; Zhang, L.; Jiang, H. An optical sensor for Cu (II) detection with upconverting luminescent nanoparticles as an excitation source. Chem. Commun. 2012, 48, 4860–4862. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Van Giap, T.; Kim, S.H.; Lee, Y.H. A novel strategy to selectively detect Fe (III) in aqueous media driven by hydrolysis of a rhodamine 6G Schiff base. Chem. Commun. 2010, 46, 1407–1409. [Google Scholar] [CrossRef] [PubMed]











| Empirical Formula | C45H26EuF9N2O9 | C11H7F3O3 |
|---|---|---|
| Formula weight | 1061.64 | 244.17 |
| Color | Colorless | Green |
| Cryst syst | Orthorhombic | Monoclinic |
| Space group | P b c a | P 21/c |
| Temperature (K) | 293(2) K | 293(2) K |
| a (Å) | 20.5707(18) | 11.927 |
| b (Å) | 19.0486(17) | 7.523 |
| c (Å) | 21.4827(19) | 13.044 |
| α (deg) | 90 | 90 |
| β (deg) | 90 | 119.09 |
| γ (deg) | 90 | 90 |
| V (Å3) | 8417.8(13) | 1022.8 |
| Z | 8 | 4 |
| ρ (g cm3) | 1.675 | 1.586 |
| μ (mm−1) | 1.590 | 0.150 |
| F (000) | 4208 | 496 |
| R1 [I > 2σ(I)] | 0.0393 | 0.0478 |
| wR2 [I > 2σ(I)] | 0.0966 | 0.1347 |
| R1 (all data) | 0.06371 | 0.0715 |
| wR2 (alldat) | 0.1129 | 0.1555 |
| GOF on F2 | 1.097 | 1.015 |
| CCDC | 1443763 | 1524762 |
| Sample | Color | Diameter (mm) | Concentration (mol/L) | After Adding Complex 1 (365 nm) |
|---|---|---|---|---|
| Sample 1 | gray | 1.4 | 0.81 × 10−6 | red |
| Sample 2 | translucent | 2.9 | 5.10 × 10−6 | red to blue |
| Sample 3 | gray | 3.3 | 1.21 × 10−6 | red |
| Sample 4 | gray | 1.8 | 0.58 × 10−6 | red |
| Sample 5 | translucent | 2.8 | 1.27 × 10−6 | red |
| Sample 6 | --- | --- | 2.00 × 10−6 | red to blue |
| water | --- | --- | --- | red |
| Sample | Complex | 4-TFC | Phen | Eu3+ | Al3+ | Color |
|---|---|---|---|---|---|---|
| a | add | green | ||||
| b | add | add | green | |||
| c | add | add | add | green | ||
| d | add | add | colorless | |||
| e | add | add | green | |||
| f | add | add | red | |||
| g | add | add | add | red | ||
| h | add | add | add | red | ||
| i | add | add | add | green | ||
| j | add | blue |
| Bond Length (Å) | Bond Length (Å) | Bond Angles (°) | Bond Angles (°) |
|---|---|---|---|
| Eu(1)–O(2) 2.3761 | Al(1)–O(2) 1.9136 | O(2)–Eu(1)–O(3) 70.2499 | O(2)–Al(1)–O(3) 89.9886 |
| Eu(1)–O(3) 2.3813 | Al(1)–O(3) 1.9189 | O(5)–Eu(1)–O(6) 69.9090 | O(5)–Al(1)–O(6) 89.1116 |
| Eu(1)–O(5) 2.3845 | Al(1)–O(5) 1.9226 | O(8)–Eu(1)–O(9) 70.9363 | O(8)–Al(1)–O(9) 90.0988 |
| Eu(1)–O(6) 2.3783 | Al(1)–O(6) 1.9255 | N(1)–Eu(1)–N(2) 61.2205 | N(1)–Al(1)–N(2) 31.6322 |
| Eu(1)–O(8) 2.3964 | Al(1)–O(8) 1.9003 | ||
| Eu(1)–O(9) 2.3370 | Al(1)–O(9) 1.9193 | ||
| Eu(1)–N(1) 2.6966 | Al(1)–N(1) 4.6949 | ||
| Eu(1)–N(2) 2.6852 | Al(1)–N(2) 5.2663 |
| Complexes | Sn | Confign | CI Codff | E/nm (eV) | Oscillator | Assignment |
|---|---|---|---|---|---|---|
| Eu complex | S6 | H-2 → L | 0.2741 | 362.17 (3.14) | 0.1253 | LMCT/MLCT/MC |
| S7 | H-1 → L + 2 | 0.3350 | 276.44 (4.49) | 0.2128 | ILCT/LMCT/MLCT/MC | |
| S8 | H → L + 3 | 0.2709 | 270.48 (4.58) | 0.4099 | ILCT/LMCT/MLCT/MC | |
| S9 | H → L + 4 | 0.3649 | 269.09 (4.61) | 0.3189 | ILCT/LMCT/MLCT/MC | |
| Al complex | S7 | H-3 → L + 1 | 0.2465 | 446.62 (2.78) | 0.1685 | ILCT/LMCT/MLCT |
| S8 | H-4 → L6 | 0.4417 | 270.08 (4.59) | 0.2344 | ILCT/LMCT/MLCT | |
| S9 | H-1 → L + 2 | 0.4838 | 266.22 (4.66) | 0.4058 | LMCT/MLCT |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Hou, Y.; Bai, J.; Shao, H.; Niu, H. Design and Synthesis of an Eu-Based β-Diketone-Sensor for the Detection of Al3+ Ions. Crystals 2017, 7, 150. https://doi.org/10.3390/cryst7060150
Yu G, Hou Y, Bai J, Shao H, Niu H. Design and Synthesis of an Eu-Based β-Diketone-Sensor for the Detection of Al3+ Ions. Crystals. 2017; 7(6):150. https://doi.org/10.3390/cryst7060150
Chicago/Turabian StyleYu, Guang, Yanjun Hou, Jinyuan Bai, Haifeng Shao, and Haijun Niu. 2017. "Design and Synthesis of an Eu-Based β-Diketone-Sensor for the Detection of Al3+ Ions" Crystals 7, no. 6: 150. https://doi.org/10.3390/cryst7060150
APA StyleYu, G., Hou, Y., Bai, J., Shao, H., & Niu, H. (2017). Design and Synthesis of an Eu-Based β-Diketone-Sensor for the Detection of Al3+ Ions. Crystals, 7(6), 150. https://doi.org/10.3390/cryst7060150