Salts and Co-Crystalline Assemblies of Tetra(4-Pyridyl)Ethylene with Di-Carboxylic Acids
Abstract
:1. Introduction
2. Materials and Methods
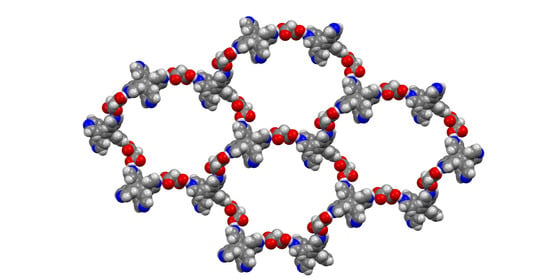
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Non-covalent interactions in the synthesis of coordination compounds: Recent advances. Coord. Chem. Rev. 2017, 345, 54–72. [Google Scholar] [CrossRef]
- Seoane, B.; Castellanos, S.; Dikhtiarenko, A.; Kapteijn, F.; Gascon, J. Multi-scale crystal engineering of metal organic frameworks. Coord. Chem. Rev. 2016, 307, 147–187. [Google Scholar] [CrossRef]
- Ok, K.M. Toward the rational design of novel noncentrosymmetric materials: Factors influencing the framework structures. Acc. Chem. Res. 2016, 49, 2774–2785. [Google Scholar] [CrossRef] [PubMed]
- Yassar, A. Recent trends in crystal engineering of high-mobility materials for organic electronics. Polym. Sci. Ser. C 2014, 56, 4–19. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.-Y. Applications of metal–organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Biradha, K.; Santra, R. Crystal engineering of topochemical solid state reactions. Chem. Soc. Rev. 2013, 42, 950–967. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Wu, C.-D. Functional porphyrinic metal–organic frameworks: Crystal engineering and applications. Dalton Trans. 2012, 41, 3879–3888. [Google Scholar] [CrossRef] [PubMed]
- Mastalerz, M. Rational design of multifunctional nanopores by mixing matching molecules. Angew. Chem. Int. Ed. 2012, 51, 584–586. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Sculley, J.; Zhou, H.-C. Metal–Organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330–1352. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.J.; Dinca, M.; Long, J.R. Hydrogen storage in metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, C.E.; Snurr, R.Q. Large-Scale generation and screening of hypothetical metal–organic frameworks for applications in gas storage and separations. In Prediction and Calculation of Crystal Structures: Methods and Applications; Atahan-Evrenk, S., Aspuru-Guzik, A., Eds.; Springer International Publishing: Cham, Switzerland, 2014; pp. 257–289. [Google Scholar]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Biradha, K.; Sarkar, M.; Rajput, L. Crystal engineering of coordination polymers using 4,4′-bipyridine as a bond between transition metal atoms. Chem. Commun. 2006, 0, 4169–4179. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission. Chem. Soc. Rev. 2011, 40, 5361–5388. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Tang, B.Z.; Liu, B. Specific light-up bioprobes based on AIEgen conjugates. Chem. Soc. Rev. 2015, 44, 2798–2811. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Gui, C.; Samedov, K.; Su, H.; Gu, X.; Li, S.; Luo, W.; Sung, H.H.Y.; Lam, J.W.Y.; Kwok, R.T.K.; et al. An acidic pH independent piperazine-TPE AIEgen as a unique bioprobe for lysosome tracing. Chem. Sci. 2017, 8, 7593–7603. [Google Scholar] [CrossRef]
- Zhou, Z.; He, C.; Yang, L.; Wang, Y.; Liu, T.; Duan, C. Alkyne activation by a porous silver coordination polymer for heterogeneous catalysis of carbon dioxide cycloaddition. ACS Catal. 2017, 7, 2248–2256. [Google Scholar] [CrossRef]
- Wang, L.; Wang, W.; Xie, Z. Tetraphenylethylene-based fluorescent coordination polymers for drug delivery. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 4263–4266. [Google Scholar] [CrossRef]
- Lustig, W.P.; Wang, F.; Teat, S.J.; Hu, Z.; Gong, Q.; Li, J. Chromophore-Based luminescent metal–organic frameworks as lighting phosphors. Inorg. Chem. 2016, 55, 7250–7256. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Huang, G.; Lustig, W.P.; Wang, F.; Wang, H.; Teat, S.J.; Banerjee, D.; Zhang, D.; Li, J. Achieving exceptionally high luminescence quantum efficiency by immobilizing an AIE molecular chromophore into a metal–organic framework. Chem. Commun. 2015, 51, 3045–3048. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.-L.; Jiang, R.; Li, Z.; Dong, Y.Q.; Du, M. Novel (4,8)-connected scu coordination framework constructed by tetrakis(4-benzoic acid)ethylene. CrystEngComm 2013, 15, 1669–1672. [Google Scholar] [CrossRef]
- Shustova, N.B.; Cozzolino, A.F.; Reineke, S.; Baldo, M.; Dincă, M. Selective turn-on ammonia sensing enabled by high-temperature fluorescence in metal–organic frameworks with open metal sites. J. Am. Chem. Soc. 2013, 135, 13326–13329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Saha, M.L.; Wang, M.; Zhou, Z.; Song, B.; Lu, C.; Yan, X.; Li, X.; Huang, F.; Yin, S.; et al. Multicomponent platinum(II) cages with tunable emission and amino acid sensing. J. Am. Chem. Soc. 2017, 139, 5067–5074. [Google Scholar] [CrossRef] [PubMed]
- Medishetty, R.; Nalla, V.; Nemec, L.; Henke, S.; Mayer, D.; Sun, H.; Reuter, K.; Fischer, R.A. A new class of lasing materials: Intrinsic stimulated emission from nonlinear optically active metal–organic frameworks. Adv. Mater. 2017, 29, 1605637. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, Z.; Lin, B.; Zhang, C.; Cao, L.; Wang, T.; Zhang, J.; Wang, C.; Lin, W. Two-Dimensional metal–organic layers as a bright and processable phosphor for fast white-light communication. Chem. Eur. J. 2017, 23, 8390–8394. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, W.; Teat, S.J.; Xu, F.; Wang, H.; Wang, X.; An, L.; Li, J. Chromophore-immobilized luminescent metal–organic frameworks as potential lighting phosphors and chemical sensors. Chem. Commun. 2016, 52, 10249–10252. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Moon, S.-Y.; Guelta, M.A.; Harvey, S.P.; Hupp, J.T.; Farha, O.K. Encapsulation of a nerve agent detoxifying enzyme by a mesoporous zirconium metal–organic framework engenders thermal and long-term stability. J. Am. Chem. Soc. 2016, 138, 8052–8055. [Google Scholar] [CrossRef] [PubMed]
- King, S.C.; Wang, H.; Arman, H.D.; Chen, B. A two-dimensional metal–organic framework composed of paddle-wheel cobalt clusters with permanent porosity. Inorg. Chem. Commun. 2016, 74, 98–101. [Google Scholar] [CrossRef]
- Deibert, B.J.; Velasco, E.; Liu, W.; Teat, S.J.; Lustig, W.P.; Li, J. High-Performance blue-excitable yellow phosphor obtained from an activated solvochromic bismuth-fluorophore metal–organic framework. Cryst. Growth Des. 2016, 16, 4178–4182. [Google Scholar] [CrossRef]
- Zhang, Q.; Su, J.; Feng, D.; Wei, Z.; Zou, X.; Zhou, H.-C. Piezofluorochromic metal–organic framework: A microscissor lift. J. Am. Chem. Soc. 2015, 137, 10064–10067. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-G.; Wang, H.; Chen, B.; Zou, Y.; Gu, Z.-G.; Zhao, Z.; Shen, L. A luminescent metal–organic framework constructed using a tetraphenylethene-based ligand for sensing volatile organic compounds. Chem. Commun. 2015, 51, 1677–1680. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, P.P.; Ditzler, L.R.; Baltrusaitis, J.; Swenson, D.C.; Tivanski, A.V.; Pigge, F.C. Semiconducting organic assemblies prepared from tetraphenylethylene tetracarboxylic acid and bis(pyridine)s via charge-assisted hydrogen bonding. J. Am. Chem. Soc. 2011, 133, 8490–8493. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, P.P.; Swenson, D.C.; Pigge, F.C. Influence of halogen bonding interactions in crystalline networks of tetraarylethylene halobenzoyl esters. Cryst. Growth Des. 2012, 12, 698–706. [Google Scholar] [CrossRef]
- Kapadia, P.P.; Widen, J.C.; Magnus, M.A.; Swenson, D.C.; Pigge, F.C. Tetrapyridyl tetraphenylethylenes: Supramolecular building blocks with aggregation-induced emission properties. Tetrahedron Lett. 2011, 52, 2519–2522. [Google Scholar] [CrossRef]
- Rudd, N.D.; Wang, H.; Teat, S.J.; Li, J. A dual linker metal–organic framework demonstrating ligand-based emission for the selective detection of carbon tetrachloride. Inorg. Chim. Acta 2018, 470, 312–317. [Google Scholar] [CrossRef]
- Zhao, S.-S.; Chen, L.; Wang, L.; Xie, Z. Two tetraphenylethene-containing coordination polymers for reversible mechanochromism. Chem. Commun. 2017, 53, 7048–7051. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Y.; Chang, Z.; Chen, L.; Bu, X.-H. A four-fold interpenetrated metal–organic framework as a fluorescent sensor for volatile organic compounds. Dalton Trans. 2016, 45, 14888–14892. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Cook, T.R.; Li, Y.; Yan, X.; Wu, D.; Shao, L.; Shen, J.; Tang, G.; Huang, F.; Chen, X.; et al. Tetraphenylethene-based highly emissive metallacage as a component of theranostic supramolecular nanoparticles. Proc. Natl. Acad. Sci. USA 2016, 113, 13720–13725. [Google Scholar] [CrossRef] [PubMed]
- Rudd, N.D.; Wang, H.; Fuentes-Fernandez, E.M.A.; Teat, S.J.; Chen, F.; Hall, G.; Chabal, Y.J.; Li, J. Highly efficient luminescent metal–organic framework for the simultaneous detection and removal of heavy metals from water. ACS Appl. Mater. Interfaces 2016, 8, 30294–30303. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.L.; Rananaware, A.; Rix, C.; Bhosale, S.V.; Latham, K. Highly fluorescent metal–organic framework for the sensing of volatile organic compounds. Cryst. Growth Des. 2016, 16, 3067–3071. [Google Scholar] [CrossRef]
- Hu, Z.; Lustig, W.P.; Zhang, J.; Zheng, C.; Wang, H.; Teat, S.J.; Gong, Q.; Rudd, N.D.; Li, J. Effective detection of mycotoxins by a highly luminescent metal–organic framework. J. Am. Chem. Soc. 2015, 137, 16209–16215. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Hu, Z.; Deibert, B.J.; Emge, T.J.; Teat, S.J.; Banerjee, D.; Mussman, B.; Rudd, N.D.; Li, J. Solution processable MOF yellow phosphor with exceptionally high quantum efficiency. J. Am. Chem. Soc. 2014, 136, 16724–16727. [Google Scholar] [CrossRef] [PubMed]
- Icli, B.; Solari, E.; Kilbas, B.; Scopelliti, R.; Severin, K. Multicomponent assembly of macrocycles and polymers by coordination of pyridyl ligands to 1,4-bis(benzodioxaborole)benzene. Chem.–Eur. J. 2012, 18, 14867–14874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Yan, X.; Saha, M.L.; Zhang, M.; Wang, M.; Li, X.; Stang, P.J. Immobilizing tetraphenylethylene into fused metallacycles: Shape effects on fluorescence emission. J. Am. Chem. Soc. 2016, 138, 13131–13134. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Yan, X.; Saha, M.L.; Niu, Z.; Stang, P.J. Hierarchical self-assembly of responsive organoplatinum(II) metallacycle–tmv complexes with turn-on fluorescence. J. Am. Chem. Soc. 2016, 138, 12033–12036. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.L.; Yan, X.; Stang, P.J. Photophysical properties of organoplatinum(II) compounds and derived self-assembled metallacycles and metallacages: Fluorescence and its applications. Acc. Chem. Res. 2016, 49, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- Pigge, F.C.; Kapadia, P.P.; Swenson, D.C. Halogen bonded networks from pyridyl-substituted tetraarylethylenes and diiodotetrafluorobenzenes. CrystEngComm 2013, 15, 4386–4391. [Google Scholar] [CrossRef]
- Gabr, M.T.; Pigge, F.C. Rhenium tricarbonyl complexes of AIE active tetraarylethylene ligands: Tuning luminescence properties and HSA-specific binding. Dalton Trans. 2017, 46, 15040–15047. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.T.; Pigge, F.C. A turn-on AIE active fluorescent sensor for Hg2+ by combination of 1,1-bis(2-pyridyl)ethylene and thiophene/bithiophene fragments. Mater. Chem. Front. 2017, 1, 1654–1661. [Google Scholar] [CrossRef]
- Gabr, M.T.; Pigge, F.C. A selective fluorescent sensor for Zn2+ based on aggregation-induced emission (AIE) activity and metal chelating ability of bis(2-pyridyl)diphenylethylene. Dalton Trans. 2016, 45, 14039–14043. [Google Scholar] [CrossRef] [PubMed]
- Bis, J.A.; Zaworotko, M.J. The 2-aminopyridinium-carboxylate supramolecular heterosynthon: A robust motif for generation of multiple-component crystals. Cryst. Growth Des. 2005, 5, 1169–1179. [Google Scholar] [CrossRef]
- Infantes, L.; Motherwell, S. Water clusters in organic molecular crystals. CrystEngComm 2002, 4, 454–461. [Google Scholar] [CrossRef]
- Batten, S.R.; Robson, R. Interpenetrating nets: Ordered, periodic entanglement. Angew. Chem. Int. Ed. 1998, 37, 1460–1494. [Google Scholar] [CrossRef]
- Shan, N.; Bond, A.D.; Jones, W. Crystal engineering using 4,4′-bipyridyl with di- and tricarboxylic acids. Cryst. Eng. 2002, 5, 9–24. [Google Scholar] [CrossRef]











© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabr, M.T.; Pigge, F.C. Salts and Co-Crystalline Assemblies of Tetra(4-Pyridyl)Ethylene with Di-Carboxylic Acids. Crystals 2018, 8, 41. https://doi.org/10.3390/cryst8010041
Gabr MT, Pigge FC. Salts and Co-Crystalline Assemblies of Tetra(4-Pyridyl)Ethylene with Di-Carboxylic Acids. Crystals. 2018; 8(1):41. https://doi.org/10.3390/cryst8010041
Chicago/Turabian StyleGabr, Moustafa T., and F. Christopher Pigge. 2018. "Salts and Co-Crystalline Assemblies of Tetra(4-Pyridyl)Ethylene with Di-Carboxylic Acids" Crystals 8, no. 1: 41. https://doi.org/10.3390/cryst8010041
APA StyleGabr, M. T., & Pigge, F. C. (2018). Salts and Co-Crystalline Assemblies of Tetra(4-Pyridyl)Ethylene with Di-Carboxylic Acids. Crystals, 8(1), 41. https://doi.org/10.3390/cryst8010041